Introduction
Light-chain amyloidosis (AL amyloidosis) results from extra cellular deposition of fibril-forming monoclonal immunoglobulin light chains. Among these fiber deposition-related organ disorders, renal lesions (amyloid nephropathy) are observed in 50~80% of patients [1]. Prognosis of AL amyloidosis is poor if untreated and is affected by the nature, number, extent of organ involvement, and the presence of concurrent myeloma [2]. To date, there is no established treatment for systemic AL amyloidosis. Conventional chemotherapy with melphalan and dexamethasone induces at least partial hematologic response in approximately 60% of patients. Recently, high-dose melphalan and hematopoietic cell transplantation (HDM/HCT) therapy has emerged as a first line treatment for AL amyloidosis [3-5]. However, clinical evidence for this therapy was mostly based on retrospective analyses and randomized controlled trials on this issue are scarce. Herein, we report a case of AL amyloidosis presented with nephrotic syndrome and clinically remitted after HDM/HCT.
Case
A 51-year-old woman visited our hospital due to generalized edema. The patient gained weight 5 kg during the last one month. Her medical history was not remarkable. Blood pressure was 109/82 mmHg, and pitting edema in both low extremities was observed on physical examination. Initial laboratory tests showed the following values: hemoglobin, 15.8 g/dL; total protein, 4.4 g/dL; serum albumin, 2.2 g/dL; total cholesterol, 295 mg/dL; aspartate/alanine aminotransferase, 18/14 IU/L; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 53 IU/L; serum creatinine, 0.8 mg/dL (corresponding to estimated glomerular filtration rate of 85 mL/min/1.73 m2); CK, 57 IU/L; CK-MB, 2.31 ng/mL; troponin-T, 0.023 ng/mL; and NT-proBNP, 663.50 pg/mL.
Electophoresis and immunofixation studies of serum and urine proteins revealed an IgM lamda type monoclonal gammopathy. Serum kappa free light chain level was 19.5 mg/L and lambda free light chain was 73.5 mg/L and their ratio was 0.27.
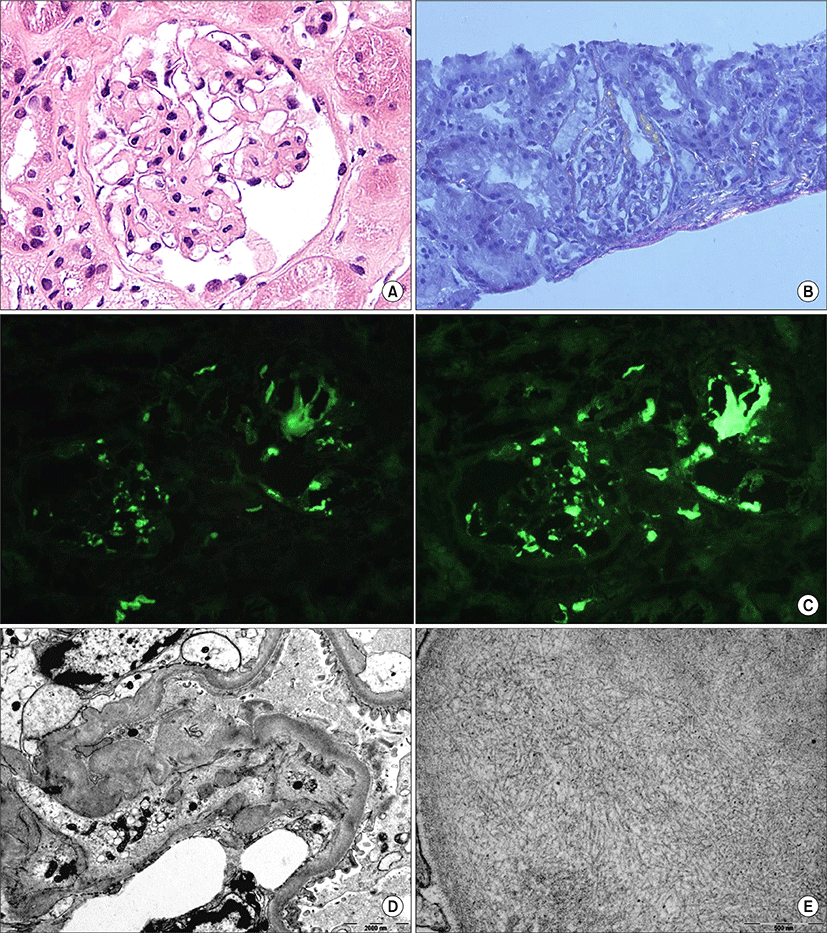
Spot urine protein creatinine ratio (UPCR) and 24 hour urinary protein excretion were 7.79 g/g, and 6.95 g/day, respectively. Other serologic tests for hepatitis B and C, human immunodeficiency virus, antinuclear antibody and antineutrophil cytoplasmic antibody were negative, and serum complement levels were within the reference range. No cardiomegaly or hepatomegaly was seen on imaging studies. On echocardiography, there was no enhancement of echogenicity in cardiac walls with preserved ejection fraction. Esophago gastro duodenoscopic and colonoscopic findings were not remarkable. Renal biopsy was performed 7 days after the initial evaluation (Fig. 1). On light microscopy, amorphous periodic acid Schiff positive deposits were observed in the glomerular capillary walls, mesangial area in glomeruli and arterioles. These materials showed an apple green birefringence by Congo red staining under polarized light. Immunofluorescence examinations demonstrated kappa and lambda chain deposits in glomeruli and arterioles without γ-, μ-, α-heavy chain deposits. Electron microscopic findings revealed diffuse fusion of foot processes, and non-branched fibers in both subepithelial and subendothelial spaces of the glomerulus. Immunohistochemical study of the biopsy tissue excluded amyloid A (AA) amyloidosis. On bone marrow examination, plasma cells accounted for 0.6% in marrow aspirate, suggesting no evidence of coexisting multiple myeloma.
The patient received autologous hematopoietic cell transplantation following the administration of melphalan at a dose of 200 mg/m2 over 2 consecutive days. No maintenance therapy was given to the patient after melphalan treatment and hematopoietic cell transplantation. Electrophoresis and immunofixation studies of serum and urine proteins 1 month after the treatment showed complete hematologic remission. Kappa free light chain level was less than 3.65 mg/L, lambda free light chain was 7.6 mg/L and their ratio was not able to calculate. No definite monoclonal band was observed in both serum and urine. During the treatment time and follow up period, there were no episodes of acute kidney injury showing unremarkable changes in serum BUN and creatinine values.
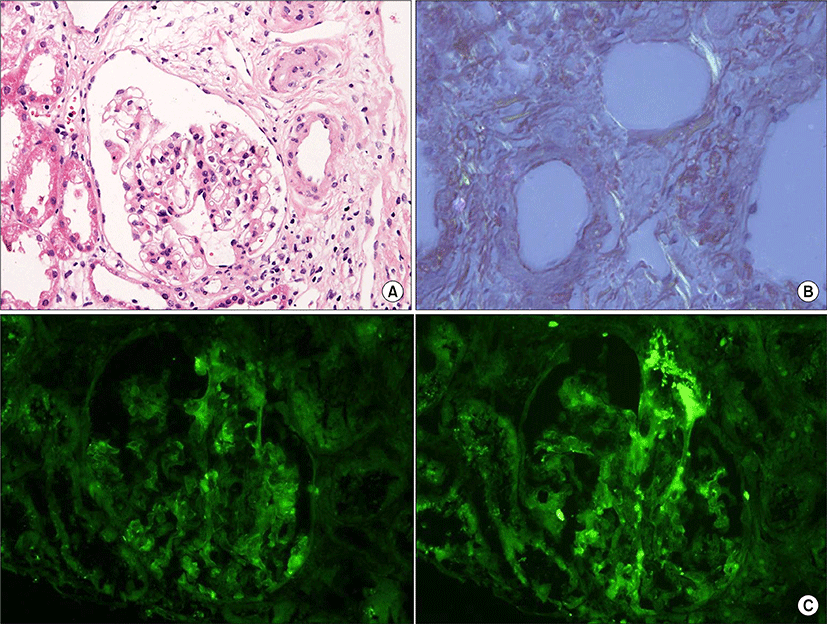
Contrary to hematologic response, nephrotic range proteinuria was decreased by 50% from the baseline value at 9 month and amount of proteinuria was normalized 17 months after HDM/HCT. A second renal biopsy was performed 25 months after the treatment. Congo red positive materials were substantially decreased compared to the initial biopsy findings. However, amyloid deposits remained in the subendothelial area of the glomerulus (Fig. 2). Spot UPCR at the last visit was 0.26 g/g. She has been in clinical remission for 28 months.
Discussion
Primary AL amyloidosis is a rare systemic disorder and its clinical features are attributed to deposition of proteins derived from immunoglobulin light chain fragments in various organs such as kidney, heart, and liver. In particular, renal manifestations include asymptomatic proteinuria, nephrotic syndrome, or progressive renal failure. End stage renal disease requiring dialysis develops in about 20% of patients with AL amyloidosis [6]. Recently, HDM/HCT has been highlighted as a potentially curative treatment for AL amyloidosis. Many studies have reported hematologic remission as well as clinical improvement in organ manifestations caused by amyloid deposition. This report describes a case of clinical remission of renal amyloidosis after this therapy.
Before HDM/HCT was introduced for treatment of AL amyloidosis, chemotherapy aimed at suppressing the underlying plasma cell clone secreting amyloid forming immunoglobulin light chain was the main therapy. However, overall survival rate was poor, as demonstrated in two randomized studies showing that a combination of melphalan plus prednisone had limited beneficial effect, with a median overall survival of 18 months, compared to 12 months in patients who received no treatment or colchicine therapy alone [7,8]. Although experiences of bone marrow transplantation for treatment of AL amyloidosis were occasionally reported in the mid 1990’s, the feasibility and efficacy of HDM/HCT was first suggested in 1998 by Ray Comenzo and colleagues [9]. Since then, many studies using the same treatment strategy have been reported. It was reported that hematological response rate was more than 60%, complete response rate and a median survival were around 40% and 4.5 years, respectively, after HDM/HCT treatment [5]. However, HDM/HCT in AL amyloidosis still remains restricted to selection of patients; generally younger than 65 years and with localized involvement less than two organs without advanced cardiac amyloidosis.
Despite complete hematologic remission and disappearance of urine M protein and proteinuria, renal histology showed that amyloid deposits remained in the subendothelial area of the glomerulus in this patient. Previous reports also demonstrated that renal amyloid depositions were not regressed even in patients showing complete remission of AL amyloidosis after successful HDM/HCT. It suggested that light chain rather than renal amyloid deposition may induce glomerular injury and increase in protein permeability in glomerulus, consequently [10]. However, careful follow up is still necessary to confirm the significance of amyloid deposition in glomerulus.
In this report, we described a case of AL amyloidosis, which presented with nephrotic syndrome and improved after HDM/HCT.