Introduction
As the population ages, the number of patients who have undergone previous coronary artery bypass grafting (CABG) requiring subsequent aortic valve replacement (AVR) will be increased. AVR is the current standard of therapy in patients with severe aortic valve disease, but is more complex in the context of prior CABG, and redo AVR after prior CABG is associated with a higher mortality than AVR as a primary procedure [1]. Redo AVR in patients with patent internal mammary artery (IMA) graft remains a challenge in terms of the critical IMA dissection and myocardial protection. Particularly in patients with patent bypass grafts, there is a 5% incidence of injury to the IMA [2]. Conventional strategy involves resternotomy, dissection and temporary occlusion of the IMA with subsequent aortic cross clamping to prevent cardioplegia wash out [3]. The problem with this procedure include that injury to the IMA graft can result in catastrophic complications. We describe a patient in whom successful conventional AVR was underwent 34 months after previous CABG with patent left IMA (LIMA) on left anterior descending artery (LAD).
Case
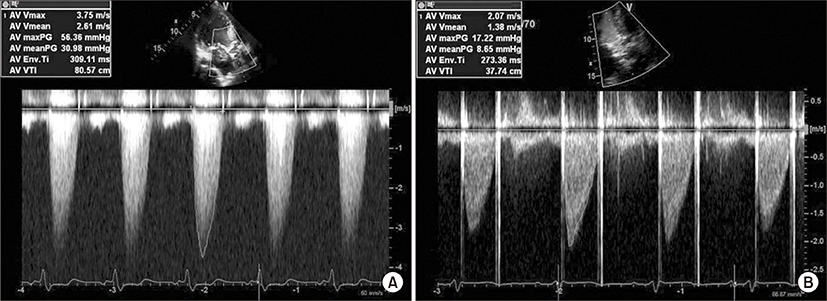
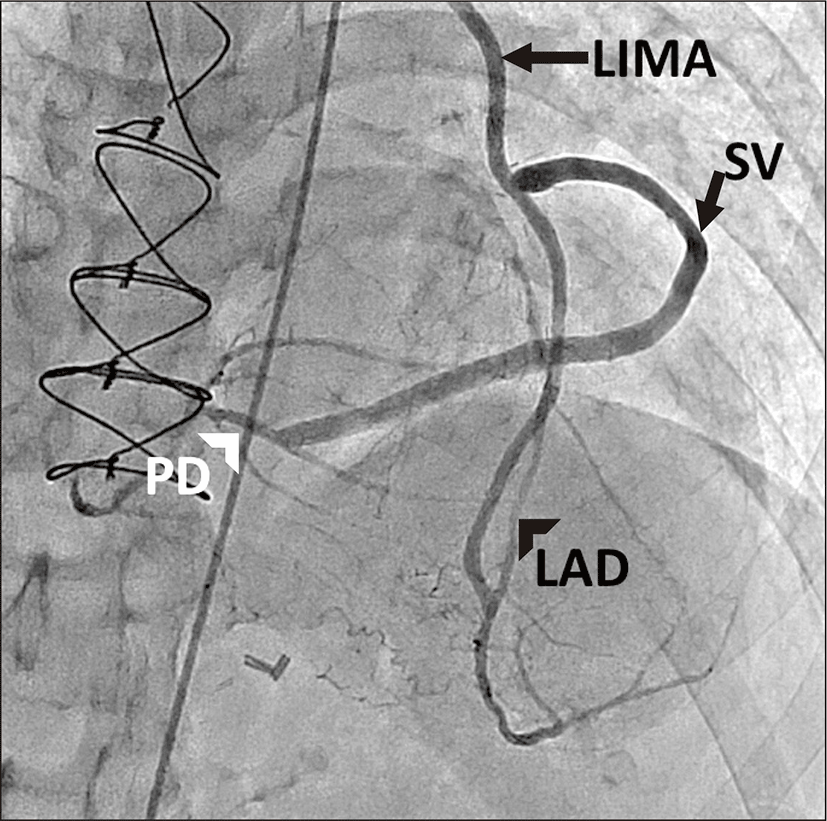
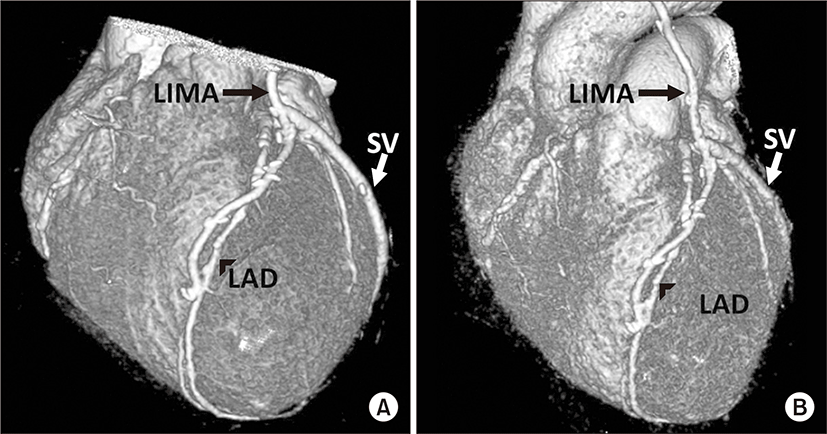
A 65-year-old man with ischemic heart disease and previous myocardial infarction underwent CABG using LIMA as conduit in December 2009. The LIMA was directly anastomosed to the distal LAD. Harvested saphenous vein was inserted to LIMA and connected to 2nd obtuse marginal branch (OM) and to right posterior descending branch (PD). His past medical history included hypertension, diabetes mellitus, and end stage renal disease on hemodialysis. He suffered chest pain and was admitted to cardiology division in August, 2012. Severe aortic stenosis with aortic valve effective orifice area (EOA) of 0.6 cm2 and transvalvular peak pressure gradient (PPG) of 56.4 mmHg were revealed on Doppler transthoracic echocardiography (Fig. 1A). In addition, coronary angiography showed patent LIMA and saphenous vein grafts (Fig. 2). AVR is indicated for the patient who was expected high mortality ratio of 12.77% by the EuroSCORE II (European system for cardiac operative risk evaluation II). Consequently, transcatheter aortic valve implantation (TAVI) was under consideration as an alternative to surgery. However, 10-year-survival rate of patients with end stage renal disease on regular hemodialysis in Korea is more than 50%, the patient’s life is expected over 75 years old. Several studies provided evidence that AVR following previous CABG is safe and has long-term effectiveness over a 10-year period. Preoperative multidetector computed tomography (MDCT) angiography was performed, presenting not only a patent LIMA and saphenous vein grafts but also structure between the ascending aorta and the sternum (Fig. 3A). Therefore the patient was planned for AVR 34 months after previous CABG on the basis of preoperative evaluation.
At surgery, standard re-midsternotomy was performed, sternum was separated from chest wall and adhesiotomy of anterior wall of aorta was done. The ascending aorta was cannulated, venous return was established with cannula placed in the right atrium. A retrograde cardioplegia line inserted in the right heart through right atrium and a vent line was placed in the left heart through the left superior pulmonary vein. The LIMA graft was dissected and clamped, simultaneously cardioplegic solution was infused via retrograde cardioplegic route. Replacement of aortic valve access exposure was provided by transverse aortotomy directed above sinus of valsalva. Excision of the aortic valve leaflets and insertion of a 21-mm bileaflet mechanical prosthetic valve (St. Jude Medical Inc., St. Paul, MN, US) were performed. The patient was uneventfully weaned from the cardiopulmonary bypass. Total bypass time was 125 minutes, and aorta cross clamp time was 73 minutes. The patient discharged without any complications during hospitalization. Postoperative 2-dimentional Doppler transthoracic echocardiography showed well functioning prosthetic aortic valve with EOA of 1.45 cm2 and transvalvular PPG of 17.2 mmHg (Fig. 1B), and MDCT revealed patency of LIMA and saphenous vein grafts (Fig. 3B). The patient was doing well.
Discussion
Redo AVR in patients with a patent IMA graft poses a high risk because of the possibility of IMA graft injury and myocardial damage. Prior studies have shown the mortality rate of 12-18% in patients who underwent AVR following previous CABG [1,4,5]. LIMA injury is associated with a mortality rate of up to 50% and a 40% chance of perioperative myocardial infarction [5]. The conventional surgical procedure in this situation involves median resternotomy and clamping of the patent bypass grafts. The advantage of clamping the patent bypass grafts is that regional myocardial rewarming and cardioplegia “wash-out” through the patent bypass grafts during cardioplegic arrest are avoided, which ensures myocardial protection. However, the dissection required to clamp the graft vessel increases the potential risk of graft injuries [6]. Various surgical techniques are presented to solve these problems. Some authors have reported on the efficacy of an alternative strategy that leaves the IMA graft undissected and unclamped. Byrne et al. [2] reported redo AVR with cardioplegia but without dissection and clamping of an IMA graft. Savitt et al. [7] reported a technique of AVR on the beating heart using antegrade continuous coronary perfusion without cardioplegia. Some authors suggest leaving the IMA graft open and perfusing the heart technique might decrease mortality rate compared with clamping the IMA graft controlled [5]. But, a larger volume of data is required for alternative techniques.
It is essential to dissect the patent bypass grafts safely and to reduce cardiopulmonary bypass time that preoperative information of the route of the graft vessels and the relationship between the graft vessels and mediastinal structures. Preoperative computed tomography has been reported to be of benefit in patients undergoing cardiac reoperations [8]. In recent years, MDCT has emerged as a highly reliable modality for the comprehensive assessment of mediastinum and bypass graft anatomy, therefore it is commonly performed for planning of reoperative cardiothoracic surgery [8]. In this case, we obtained the information about anatomy of the bypass grafts and other mediastinal structures from MDCT. It is also useful modality to evaluate patency of graft vessels and coronary arteries after the operation.
Increasing patient age and comorbidities may increase operative mortality [9]. Therefore, less invasive percutaneous approaches have been developed. In the last decade, there have been significant advances in percutaneous aortic valve interventions. The PARTNER trial demonstrated a significant survival advantage for TAVI in high risk patients with aortic stenosis compared with medical management alone [10], and the subsequently published randomized comparison of TAVI versus open AVR in high risk patients (PARTNER A) showed similar survival at 1-year follow-up [11]. Consequently, TAVI may now become the treatment of choice in patients in whom open AVR confers too great an operative risk. TAVI can nullify some of the operative risks associated with prior CABG. Therefore, TAVI is feasible option in patients with aortic stenosis and prior CABG and potentially negates the risk associated with revision sternotomy and damage to patent grafts. TAVI is considered as an attractive option in the population of high risk patients with aortic stenosis and previous CABG [12]. However, there is no prospective study comparing TAVI with surgical AVR in moderate or low risk populations. There are data that AVR following previous CABG is safe and has long-term effectiveness [13,14]. Although further prospective studies comparing TAVI and AVR might be warranted in patients with previous CABG, the wealth of evidence currently supports surgical AVR.
As the population ages, an increasing number of patients who have undergone previous CABG will require subsequent AVR. Generally AVR following previous CABG is a safe procedure in experienced centers. In patients undergoing AVR after previous CABG with patent bypass grafts, MDCT helps to define the anatomy of the bypass grafts and their relationships with other mediastinal structure, and physicians and surgeons should carefully plan the AVR procedure to achieve a favorable outcome.