Introduction
Uterine tumors resembling ovarian sex-cord tumors (UTROSCT) are very rare tumors originating from the uterine body first named by Clement and Scully in 1976 [1]. They described 14 cases of UTROSCT and classified them into two groups according to clinical and histologic features. Each group is classified based on the amount of sex-cord like elements present. Group 1 tumors are endometrial stromal tumors with sex-cord-like element with more frequent metastasis and relapse rates, whereas group 2 tumors are predominantly consisted of sex-cord elements and has lower rates of metastasis and recurrence [2,3].
Clinically, these tumors occasionally secrete hormones that cause symptoms of abnormal vaginal bleeding. The UTROSCT are usually diagnosed clinically as endometrial polyps or leiomyomas, and are often confirmed through immunohisto-chemical or ultrastructural studies after surgery [4,5].
Both UTROSCT and endometrial stromal tumors with sex-cord-like elementare masses that occur well in the uterine fundus, and macroscopic pathology findings show that polypoid masses are commonly projected into the uterine cavity. The mean uterine weight and size are 485 g and 6.2 cm [3,6]. Microscopic findings show various architectural patterns such as an anastomosing cord 1 to 2 cells wide, broad trabeculae, small nests, and sertoliform or retiform tubular structures, Call-Exner-like bodies and diffuse sheets of uniform granulosa cell tumor-like area. Neoplastic cells mainly have round to ovoid nuclei, little nuclear pleomorphism, inconspicuous nucleoli, scant, indistinct eosinophilic cytoplasm and are small in size [6,7]. This tumor shows a variety of immunochemical profiles of sex cord (calretinin, inhibin, and CD99), epithelial (keratin and epithelial membrane antigen [EMA]), smooth muscle lineages (actin and desmin), and miscellaneous marker (CD10, estrogen receptor, and progesterone receptor) expression. CD99 (92.9%), calretinin (90.5%), CD56 (90%), progesterone receptor (88.9%), vimentin (88%), keratin (84.2%) were expressed very high, whereas CD10 (31.8%), EMA (18.8%) was low (Table 1) [3,6,7]. Shah et al. [8] described that the immunohistostaining results for inhibin and calretinin are significant in the diagnosis of UTROSCT.
The gold standard of UTROSCT treatment is simple hysterectomy with or without bilateral salpingo-oophorectomy. Anastrozole (aromatase inhibitor) and megestrol acetate are used in patients with recurrent UTROSCT [9].
Differences in surgical methods showed no significant difference in disease-free survival (DFS). However pelvic pain, histologic subcategory, tumor size, disease spread and lymphovascular space invasion significantly reduced DFS. Postoperative overall survival rates of UTROSCT were good at 1 year (97.0%), 2 years (97.0%), and 5 years (97.0%). DFS rates were 1 year (97.0%), 2 years (92.7%), and 5 years (69.7%) after surgery. DFS rates for type 1, type 2 and unclassified group differences were 2 years (23.8% vs. 100% vs. 100%) and 5 years (0% vs. 50% vs. 81.8%) [3].
In this study, we experienced two types of UTROSCT found in patients with different symptoms. Unlike group 1 UTROSCT cases that were previously reported, we observed a subserosal type of ruptured necrotic mass with the presence of hemoperitoneum in a young female patient who experienced abdominal pain after the delivery.
Case
The patients provided written informed consent for the publication and the use of their images.
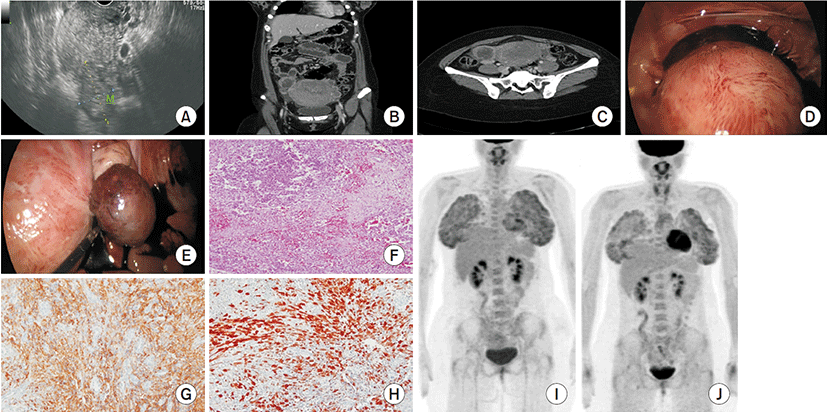
A 29-year-old Korean woman, gravida 1, para 1, was admitted to our hospital because of severe abdominal pain. The patient had vaginal delivery 6 days prior, and had no specific medical history. Laboratory tests performed after admission were within normal range. Transvaginal sonography showed a well-defined hyperechoic ovoid mass in contact with the surface of the uterus (Fig. 1A). CT showed a low-density mass of 4.3 cm in contact with the uterus and fluid collection within the abdominal cavity (Fig. 1B, C).
Clinically, explorative laparoscopy was performed under the suspicion of degenerated subserosal leiomyoma or right ovarian torsion. During the operation, ruptured necrotizing subserosal mass of the uterus and a large amount of dark blood was observed in the abdominal cavity (Fig. 1D, E). There were no lesions in both ovaries and fallopian tubes. Under laparoscopic guidance, blood and the subserosal mass were removed.
On macroscopic examination, the mass was approximately 6.5×6.5×1.5 cm and consisted of a hard solid mass with a grayish white capsule. The cut surface was heterogenous with multiple scattered old hemorrhages and white solid areas.
On microscopic examination, focal sex-cord-like differentiation was seen in the hemorrhagic background of proliferation of endometrioid stromal tumor cells (×100) (Fig. 1F). Tumor cells are immunoreactive for CD10, used as a miscellaneous marker for UTROSCT (×200) (Fig. 1G). Also the tumor cells immunostained positive with antibodies that recognize sex-cord elements (calretinin) (×200) (Fig. 1H). Thus the tumor was pathologically confirmed as group 1 UTROSCT.
We performed a PET-CT scan in order to confirm the presence of metastasis to other organs in clinical correlation to results such as rupture of the mass and pathologic examination. The PET-CT scan (Fig. 1I) showed an absence of other metastasis. The patient was recommended to have total abdominal hysterectomy and bilateral salpingo-oophorectomy surgery, but refused because she was considering additional pregnancy. After meeting with the patient, an outpatient progress observation was taken. After 3 months, we re-performed a PET-CT scan (Fig. 1J) and confirmed no metastasis and recurrence. The patient no longer wanted future pregnancy, and thus, have total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic lymphadenectomy were performed at a different hospital. Post-surgery biopsy showed no metastasis and is currently on outpatient progress observation.
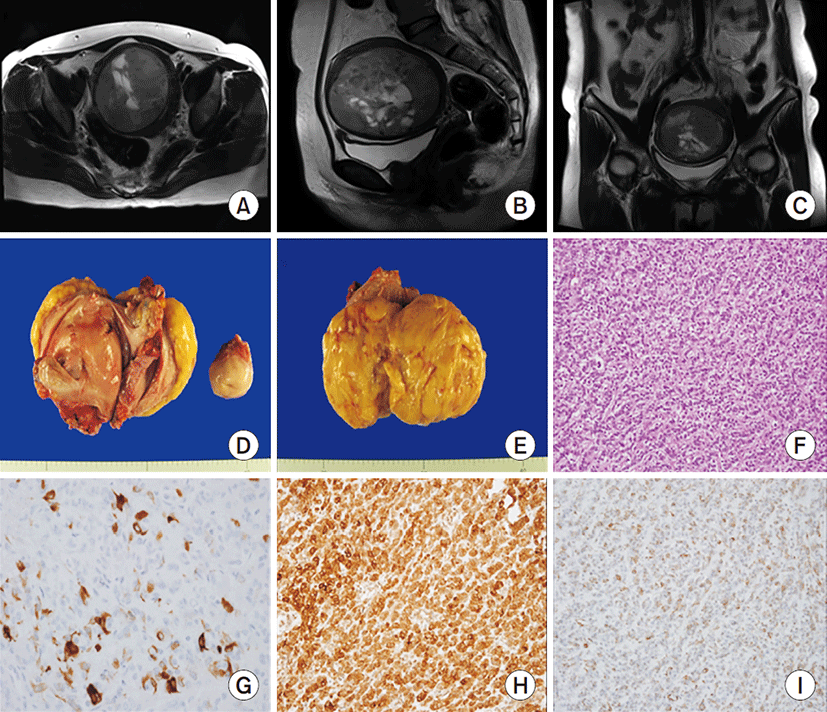
A 49-year-old Korean woman, gravida 2, para 2, visited our hospital due to vaginal bleeding that began 3 weeks ago. A heterogeneously enhancing solid mass with a clear margin was observed in the uterus on MRI. On T2-weighted images, the non-enhancing portion of the tumor was seen as necrotic or cystic tissue (Fig. 2A-C). Hysterectomy and bilateral salpingo-oophorectomy were performed under the diagnosis of degenerated uterine myoma. Macroscopic examination showed the presence of a large yellow mass measured as 9.0×7.5 cm. The cut surface of the mass was soft, yellow, and homogeneous (Fig. 2D, E).
Upon microscopic examination, the microphotograph revealed cord-like, trabecular, and nested growth patterns of epithelioid cells. The tumor cells showed ovoid nuclei with irregular contours and occasional nuclear grooves, resembling granulosa cells of ovarian granulosa cell tumors (hematoxylin and eosin staining, ×200) (Fig. 2F). The tumor cells stained positive with antibodies that recognize sex-cord elements (inhibin and CD99) (Fig. 2G, H) and myoid differentiation (smooth muscle actin) (Fig. 2I), revealing positive expression. Thus the tumor was pathologically confirmed as group 2 UTROSCT.
There was no local recurrence or metastasis one year after discharge.
Discussion
After the classification of UTROSCT in two different groups using clinical and histologic findings by Clement and Scully in 1976 [1], profound research has been performed to diagnose and treat UTROSCT. The main symptom of UTROSCT is known as post-menopausalvaginal bleeding and abnormal menstruation [10,11].
These UTROSCT usually have symptoms of abnormal vaginal bleeding or enlarged uterus in middle age women (average, 50 years). Both group 1 and group 2 have a polypoid mass that grows into the uterine cavity, involving the endometrium [6]. In previous studies, 38 case reports were reviewed. UTROSCT were most commonly of the intramural type followed by the submucosal type, and the subserosal type was very rare [12]. In this case, subserosal type UTROSCT with pain was misdiagnosed as degenerated subserosal leiomyoma or ovarian torsion. Patients with an adnexal mass with pain should undergo differential diagnosis such as ovarian mass, subserosal myoma, and subserosal type UTROSCT. Specific ultrasound findings of UTROSCT are important for diagnosing UTROSCT showing various symptoms, morphology, and characteristics only for UTROSCT. Unfortunately, UTROSCT are not reported in many cases yet, so the specific sonographic features are not known. Therefore, differential diagnosis with endometrial malignancy, endometrial hyperplasia, pyometra, hydatidiform mole, and incomplete abortion is necessary if polypoid and echogenic endometrial masses are seen in the uterus [13].
Group 1 of UTROSCT have features similar to low grade endometrial stromal cancer and group 2 is known to show benign characteristics [2,14]. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are the main treatments for both groups, but uterine preservation surgery is performed in patients who want to preserve fertility [15]. In this case, only the tumors were removed under diagnosis of uterine leiomyoma in UTROSCT patients, but additional surgery was required post pathologic examination after the surgery. As a result, additional pathologic examination is necessary to distinguish other diseases such as UTROSCT after surgery in patients with suspected uterine leiomyoma with abdominal pain.
In conclusion, we performed tumor removal in a reproductive age woman diagnosed with group 1 UTROSCT, and then performed additional surgery. Also a woman with abnormal vaginal bleeding diagnosed as group 1 UTROSCT underwent curative surgical treatment. Like our case, the patient may be diagnosed with UTROSCT after surgery and may need additional treatment or evaluation. Therefore, it is essential to confirm pathologic results in patients clinically diagnosed with leiomyoma. If the patients have symptoms such as abnormal vaginal bleeding and abdominal pain with uterine mass, the possibility of UTROSCT should also be considered.
Furthermore, because of the rarity, preoperative radiologic and laboratory diagnostic tools for UTROSCT are yet to be established. Therefore, we conclude that further researches are needed to improve the rate of diagnosis.