Introduction
The Austrian dermatologist Benjamin Lipschutz first described acute genital ulcers in adolescent girls in the absence of any evidence of sexually transmitted infections [1]. Lipschutz ulcer is an entity that presents as an acute ulcer in the labia minora or majora, introitus, fourchette, or vestibule, accompanied by flu-like systemic symptoms such as fever, tonsillitis, and/or lymphadenopathy.
Lipschutz ulcer is a diagnosis of exclusion. It mimics a wide spectrum of diseases ranging from infective causes (syphilis, herpes genitalis, and chancroid), to inflammatory conditions (Behcet disease and Crohn disease), as well as trauma. After ruling out sexually transmitted infections, inflammatory conditions, and systemic illness, Lipschutz ulcer diagnosis is established according to five major and one of two minor criteria (Table 1) [2].
The etiology of this disease remained obscure until the 1960s, when the first clues revealed the involvement of viruses or bacteria. Studies have since then revealed that Lipschutz disease is associated with viruses such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), and mumps virus, as well as bacteria such as Salmonella, Mycoplasma pneumoniae, and M. fermentans [3].
Case
An 11-year-old girl was referred to our hospital because of fever, myalgia, and ulcer in her genital area. She had no history of recurrent oral or genital aphthous lesions. Four days before admission, she started experiencing fever (max 39.7℃), sore throat, myalgia, and mild dry cough. Then she started experiencing intense pain and burning sensation in her genital area on day 2 after the onset of fever.
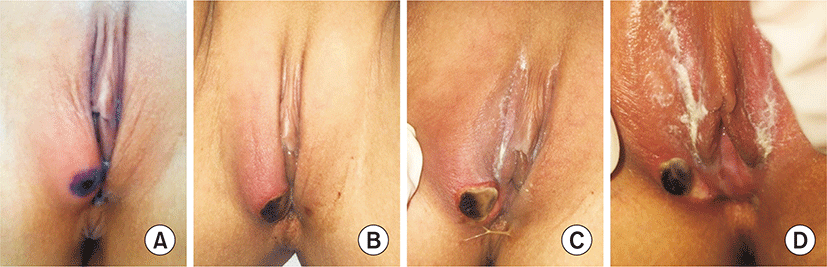
On admission, she had fever, myalgia, and chills, but no diarrhea or abdominal pain. Physical examination revealed a painful ulcer with a single necrotic eschar (1.0 cm×1.5 cm) located inside the right side of the labia majora (Fig. 1). This ulcer was severely tender upon palpitation, and no oral ulcers or swollen lymph nodes were detected. She showed no symptoms related to gastrointestinal, neurologic, or orbital lesions, and furthermore, she was not sexually active and had no previous history of similar ulcers. She was initially treated with intravenously administered antibiotics and local wound care with antiseptics.
Analytical test results showed elevated C-reactive protein (0.86 mg/dL; normal range, <0.5 mg/dL). Her complete blood count and liver enzyme values were normal at admission. C-reactive protein levels dropped to 0.57 mg/dL after starting treatment with azithromycin and ampicillin-sulbactam intravenously. Her serum test was negative for herpes simplex virus (HSV), CMV, EBV, parvovirus, mumps virus, varicella-zoster virus, hepatitis B, or hepatitis C. Furthermore, her serum anti-streptolysin O was normal, and human immunodeficiency virus and syphilis serologic tests were all negative. Her blood and urine cultures were negative. Antibodies against antineutrophil cytoplasmic antibodies-proteinase, and antineutrophil cytoplasmic antibodies-myeloperoxidase were all negative. Antinuclear antibody screening and titer were all negative. Her immunoglobulin G, A, and M were normal. Furthermore, her serum complement factor 3 and 4, and CH50 tests were normal. Her nasopharyngeal secretion was tested for a panel PCR for respiratory virus, and the results were all negative for adenovirus, respiratory syncytial virus A and B, influenza A and B, parainfluenza 1-4, metapneumovirus, rhinovirus A, B, and C, bocavirus, enterovirus, and human coronaviruses 229E, OC43, and NL63. The results of her stool PCR for gastroenteritis related virus and bacteria were all negative. Her serum M. pneumoniae IgM test was with in borderline range (0.93; borderline, 0.91 to 1.09). Four days after admission, her serum M. pneumoniae IgM was still borderline, although the titer was slightly elevated (0.96; borderline, 0.91 to 1.09). PCR test and culture of her vaginal discharge were negative for Chlamydia trachomatis, Neisseria gonorrhea, Trichomonas vaginalis, Treponemapallidum, HSV type I and II, and Candida albicans. Only normal floras were checked in the wound culture. Soft tissue sonography of her genital ulcer revealed that there was no abscess pocket in the swollen region of the right major labia.
She became afebrile after 5 days of treatment starting with azithromycin and ampicillin-sulbactam. Treatments during hospitalization included supportive local care, pain and fever control with oral analgesics and topical steroid ointment. She was discharged from hospital in good condition after a total hospital stay of seven days. After a 2-week follow-up, her genital necrotic eschar dropped off and showed re-epithelization of the vulvar skin (Fig. 2). Six months later, she had no recurrence of the ulcer.
This study was a retrospective study, and it was approved by the institutional review board of the Myongji Hospital (MJH2020-01-008).
Discussion
A Lipschutz ulcer is defined as a vulvar ulcer with no identifiable etiology, based on clinical, histopathologic, serologic, and microbiologic findings. These ulcers are characterized by sudden painful genital ulceration, occurring mostly in young virgin girls, accompanied with malaise, fever, and other systemic symptoms. These distressing symptoms are rare and may be presented to dermatologists, gynecologists, or pediatricians. It is often misdiagnosed as a sexually transmitted disease or even taken as a sign of child abuse. Some clinicians have noted that the presenting symptoms of a Lipschutz ulcer may be confused with sexual abuse, a terrible diagnosis, especially in children, and can lead to unnecessary investigations, treatments, and anxiety within the family [2]. Generally, the natural course is benign, with spontaneous regression occurring within just a few weeks.
Despite its long history, this condition is not well recognized, and its root cause is still poorly understood. Over 70% of all reported patients were ultimately diagnosed with idiopathic vulvar ulcers. Some cases were reported to be preceded or accompanied by systemic infections such as infectious mononucleosis (EBV), influenza, CMV infection, mumps, paratyphoid fever, or mycoplasma pneumonia. Other reported preceding illnesses include viral gastroenteritis, viral upper respiratory tract illness, and streptococcal pharyngitis. The pathogenesis of Lipschutz ulcer is still an enigma. It could develop from a hematogenous spread or autoinoculation, although one hypothesis suggests that it could arise from a hypersensitive reaction to a viral or bacterial infection, leading to the deposition of immune complexes in the dermal vessels, which in turn activates the complementary systems, resulting in micro-thrombi formation and subsequent tissue necrosis [4,5].
Extensive laboratory work is often conducted, creating a great deal of anxiety in both patients and parents, while also generating enormous and unnecessary medical expenses. A limited infectious evaluation, including serologies (EBV, CMV, influenza, and mycoplasma) may be considered in patients with systemic symptoms, including persistent fever, extreme fatigue, swollen lymph nodes, and/or persistent or severe sore throat. Furthermore, testing for human immunodeficiency virus, syphilis, HSV, and hepatitis B and C was negative in all reported patients. In addition, through careful physical examination and history, it is imperative to rule out other causes of more frequent genital ulcers, particularly sexually transmitted diseases, and to discuss the main differential diagnoses, such as Behcet disease or cutaneous localization of Crohn disease. Routine bacterial and fungal cultures reveal skin flora or non-pathogenic bacteria and do not offer any additional benefit [6]. Skin biopsies are also not recommended as a first-line investigation because they only indicate non-specific dermal infiltrate of mixed inflammatory cells in most patients. A skin biopsy from the edge of the ulcer is advised for ulcers lasting longer than 4 weeks [7].
Lipschutz ulcers are self-limiting and generally recover spontaneously. Treatments are mainly aimed at relieving pain and healing of the ulcer. Therefore, patients in many case reports were treated symptomatically with analgesics, topical steroids, and antibiotics. In addition, treatment with a brief course of systemic corticosteroid (0.5 mg/kg of prednisolone for 1 to 2 weeks) may help in healing severely painful, multiple, or necrotic ulcers [8,9]. Antiviral therapy can be chosen if HSV was thought, but patient’s ulcer lesions didn’t look like genital HSV (vesicles), we didn’t use antiviral agents [10]. Healing occurs within 6 weeks, without scarring. The mean healing time was reported to be16 to 21 days (range, 5 to 52 days) [11,12].
A case in South Korea, a sexually inactive 16-year-old girl with fever, oral ulcers, genital ulcers was diagnosed with Bechet disease. She had no history of oral or genital ulcers and Bechet disease. Serologic tests included VDRL, EBV, HSV IgM, HLA-51 were negative. After 4 weeks, ulcers were disappeared. During 4-month follow-up, there was no recurrence of ulcer [13]. Another case in South Korea, a 13-year-old previously healthy girl, with no personal history of recurrent oral or genital aphthous lesions, presented with sudden onset of two painful vulvar ulcers and fever. Serologic studies were all negative. She was discharged from hospital in good condition without pain after a total hospital stay of 8 days. The 3-week follow-up showed total resolution and re-epithelization of the vulvar lesion. No recurrences occurredduring the following 6 months [14].
To conclude, we presented a case of Lipschutz ulcer in a sexually inactive 11-year-old girl in South Korea. Lipschutz vulvar ulcer is a rare clinical entity due to the deleterious, extremely painful ulcerations among sexually inactive peripubertal girls. It is often misdiagnosed, over-investigated, and under-reported. Recognition of Lipschutz ulcer is important to the extent that patients receive appropriate and timely treatment,in addition to prognostic counseling. More than 70% of Lipschutz ulcers are idiopathic. No formal treatment guidelines for Lipschutz ulcer exist. Therefore, more studies are necessary to determine the organisms associated with Lipschutz ulcer and the relevant guidelines for confirming diagnosis.