Introduction
Socially and psychologically, fecal incontinence (FI) is a tragedy. Normal bowel continence is a complicated process including the anal sphincters, pelvic floor, stool volume, rectal compliance, and neurologic function. It's possible for FI to occur if even one of these things goes wrong [1].
Due to the humiliating nature of FI symptoms, many are reluctant to disclose their illnesses to doctors. Because individuals underreported their symptoms, the true prevalence of FI was unclear. Consequently, the prevalence of FI has fluctuated widely between 2% and 20% [2,3]. FI prevalence varies with age. The prevalence of FI rose with age, from 2.91% in individuals aged 20% to 29% to 16.16% in those aged 70 and older [2]. Extremely high incidence of FI are found among nursing home residents. The prevalence of FI is considerably higher than 50% among nursing home residents [3,4]. Currently, breakthroughs in sphincter-preserving surgery for rectal cancer result in incontinence, which is a symptom of the low anterior resection syndrome [5,6]. Consequently, the old population is anticipated to grow. Consequently, the prevalence of FI is anticipated to increase. The treatment of FI ranges from non-invasive strategies, such as lifestyle modifications, medical pills, sphincter augmentation with injections, and biofeedback therapy (BFT), to invasive sacral nerve stimulation (SNS) and surgical interventions [7–9].
Given that the number of patients with FI is likely to rise, it is crucial to identify the treatments now in development and to establish treatment indications. In this review, we therefore considered BFT and surgical procedures for FI.
Definition and Etiology of Fecal Incontinence
Rome IV diagnostic criteria described FI as "recurrent uncontrolled flow of feces in an individual with at least 4 years of developmental age." [10]. With the release of Rome IV diagnostic criteria in 2016, the definition of FI was modified. Rome III separated structural and neurogenic factors for functional FI. However, Rome IV defines FI as the involuntary passage of solid or liquid stool, regardless of the underlying reason. This indicates that there are several, overlapping causes of FI, and that patients with purely psychosocial or bowel habit disorders are uncommon. Additionally, there was no effective treatment advice based on the differentiation between causes of FI. The second modification to the diagnostic criteria for FI concerns the definition of event frequency. FI is defined as the development of symptoms at least six months prior with two to four episodes of FI over four weeks. In contrast, Rome III required only one FI event within the previous three months [11]. According to several researchers, this restricted modification negatively impacts the quality of life for some patients. This stringent frequency threshold resulted in a lower prevalence of FI compared to prior estimations. These excluded patients have a diminished life quality [12]. Therefore, additional research is necessary to provide a precise definition of FI in Rome V. Damages to the continence process, including the anal sphincter, bowel habits, rectal compliances, pelvic floor muscles, and rectal sensibility, might cause FI. Multiple variables cause FI in 80% of individuals [13]. Several studies have identified FI risk factors (Table 1) [13–15]. Age and gastrointestinal (GI) diseases that cause stool alterations are risk factors for FI. Aging impacts the etiology of FI, resulting in a decrease in pelvic floor muscle mass and an increase in co-morbidities that compromise the mechanism of continence. In addition, sphincters may be altered by fibrosis and thickening as a result of decreased resting tone, with the external anal sphincter's thinning resulting in a decrease in anal pressure. Moreover, diminished rectal sensitivity, rectal compliance, and rectal capacity impede anal sensory, motor, and rectal reservoir function in the aged [16]. Inflammatory bowel disease and irritable bowel syndrome characterized mostly by diarrhea are risk factors for FI. However, any GI condition that causes frequent bowel movements or a change in bowel habits can lead to FI [17]. And the structural causes of FI are obstetric injuries [18], anorectal surgery [19,20], rectal prolapse [21], and anal intercourse [22]. Puborectal or anal sphincter dysfunction may result in FI. These injuries are possible during spontaneous vaginal birth or forceps-assisted surgical delivery. Ischemia, laceration, and compression of anal muscles caused by spontaneous labor and delivery contribute to incontinence [23]. Even in the absence of complications, up to 35% of first-time vaginal deliveries may result in hidden sphincter injury. Risk factors for FI included forceps-assisted surgical delivery, occipitoposterior presentation of the infant, and protracted labor [16]. Patients who underwent anorectal procedures such as hemorrhoidectomy, sphincterotomy, and fistula surgery were frequently discovered to have FI [24]. Rectal prolapse results in incontinence as a result of rectal mucosa transporting feces via the anus. And chronic rectal prolapse causes anal sphincter dilatation and dysfunction [21].
Diagnosis
As with any disorder, FI requires a more thorough medical history. Patients are hesitant to discuss their incontinence problems regularly due to their feelings of embarrassment. The history of a patient's symptoms must be taken with care, thoroughness, and sensitivity [25]. Clinicians should question thoroughly about the patient's underlying condition, medications, and surgical history. In addition to obstetric and anorectal surgery, which are directly associated, it is vital to examine the patient's surgical history, including procedures that appear unrelated, such as cholecystectomy and spinal surgery. Additionally, it is vital to consider drugs for diarrhea and constipation. Changes in bowel, feces, and gas patterns must also be evaluated. Bowel diaries and the Bristol stool form scale can help describe the severity of FI symptoms [26].
Scoring FI systems have been utilized for objective evaluation and evaluation of therapy outcomes. The Wexner Cleveland Clinic Score [27] and the St. Mark's Incontinence Score [28] in conjunction with the FI Quality of Life (FIQL) scale [29] accepted by the American Society of Colon and Rectal Surgeons (Table 2). CCF is the most widely used historical scale. The CCF comprises of five questions, and the sum of each score allows for the diagnosis of FI. The FI Quality of Life scale analyzes performance across four broad categories. Lifestyle, coping/behavior, depression/self-perception, and shame are these categories [29]. St. Mark's score is determined by four criteria: solid stool incontinence, liquid stool incontinence, gas, and lifestyle changes [28]. There are various scales that can be used to assess the severity of FI symptoms. All of these measures have been employed to diagnose FI, despite the fact that only one has been identified. The physical examination involves a perineal skin inspection for scars from trauma, childbirth, past surgery, fistula, or hemorrhoids, as well as a digital rectal exam. The perineal body of female patients was palpated to see if it has been thinning. The Valsalva technique is a strong attempt to exhale against a closed airway, typically performed by closing the mouth and pinching the nose while exhaling. This action is beneficial for distinguishing rectal prolapse. Examining the perianal sensation to pinprick and the ano-cutaneous "wink" reflex will provide a straightforward assessment of neurologic function. A digital rectal examination can detect a fistula or hemorrhoid tumor or lesion. With the doctor's finger inserted into the patient's anus, the patient is instructed to squeeze the sphincter, which provides a general assessment of both resting tone and voluntary squeeze. Colonic exams such as colonoscopy and anoscopy are also helpful to diagnose FI [30].
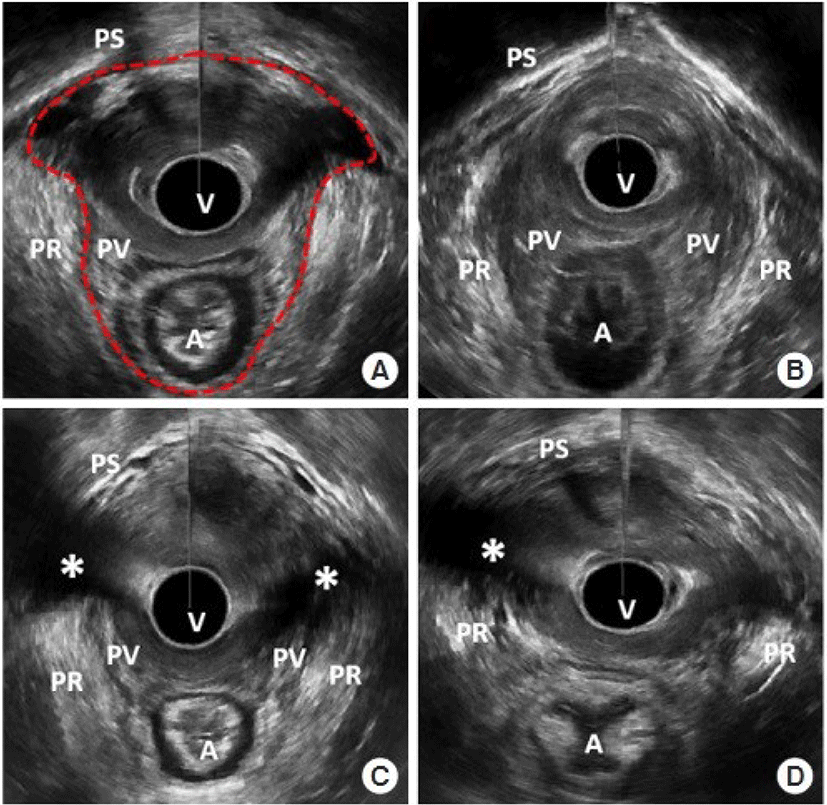
Ultrasound imaging of the anorectum is the most sensitive method for evaluating the anal complex for the existence of any defect or lesion [30]. Endoanal ultrasound imaging permits the categorization of lesions according to their whole or partial thickness and degree of disruption. A common application of anal ultrasonography prior to anus surgery is to identify the location and extent of anal sphincter problems. Ultrasonography of the 3D pelvic floor revealed anal sphincter damage and levator ani muscle avulsion in all groups. In addition, defecography and pelvic MRI are tests that can detect rectoceles and internal rectal prolapse, as well as see pelvic floor muscle activity during defecation (Fig. 1) [31,32].
Anorectal manometry evaluates the resting and squeezed anal sphincter function of patients [25]. Manometry measures muscular strength and reservoir function, which includes anorectal sensation, volume tolerance, and rectal compliance [33]. In the high-pressure region of the rectum, the recto-anal inhibitory reflex is evaluated via fast balloon inflation and deflation. A diminished recto-anal inhibitory reflex is indicative of a neurological disorder. Pudendal nerve terminal motor latency is used to diagnose pudendal neuropathy or systemic diseases such as diabetes and chemotherapy. Electrical impulses are administered to the pudendal nerve after an electrode is affixed to the examiner's finger and directed toward the ischial spine. The response time at the external anal sphincter level is measured. Normal reaction time is 2.0±0.2 milliseconds.
Electromyography employs a concentric needle electrode to record the electrical activity produced by the anal sphincter muscle fibers. Encircling the anal canal, continuous recordings of the motor units are taken. This test is used to map the external sphincter and neuromuscular function.
Treatment
FI treatment can be categorized into non-surgical and surgical approaches. We stated non-medical treatment methods that excluded drug treatment.
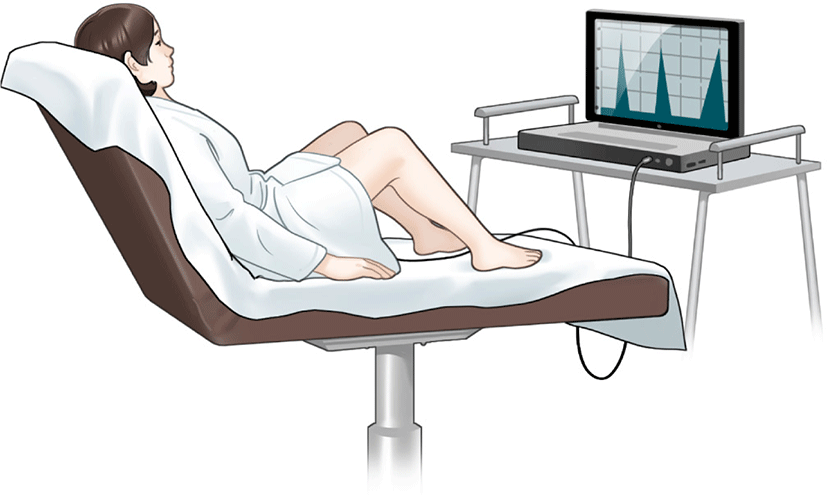
BFT involves the use of electronic devices to monitor physiologic activities, followed by the transmission of a visual or auditory signal to the patient (Fig. 2). It makes patients sensitive to rectal distension and reinforces correct sphincter contraction. Anorectal manometry or electromyography are utilized to perform the BFT. BFT consists of rectal sensitivity training, pelvic floor muscle strength training, and coordination training involving the insertion of an air or water-filled balloon into the rectum of patients. Patients feel the distension of the balloon and are instructed to squeeze their pelvic floor muscles to determine the sensation of rectal filling and prevent leakage [34].
In 2012, a Cochran review of 21 studies involving a total of 1,525 patients did not find evidence that pelvic floor muscle exercise and BFT were superior to other treatments. However, it was discovered that BFT was more effective than pelvic floor muscle exercises alone, such as Kegel, for patients whose conservative treatments had failed [35]. In a recent randomized controlled trial, patients with FI responded similarly to oral placebo plus education only, oral placebo plus anorectal manometry-assisted biofeedback, loperamide plus education only, and loperamide plus anorectal manometry-assisted biofeedback [36]. BFT is a noninvasive, first-line treatment option for highly motivated patients whose medical treatments have failed [37].
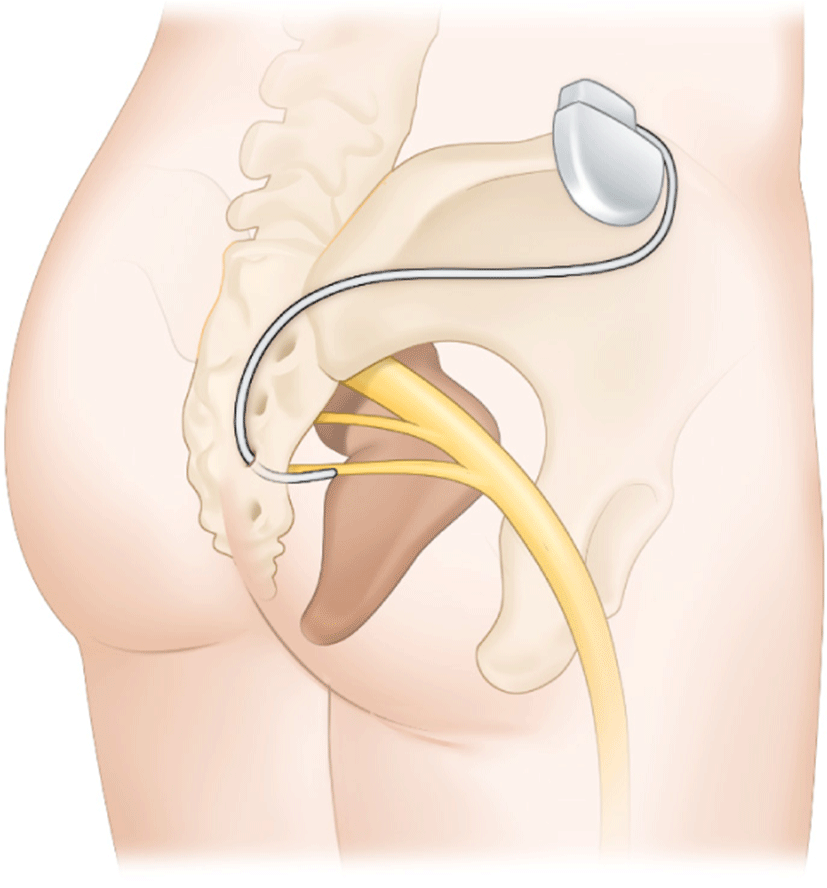
SNS is a treatment for patients whose conservative treatment has failed. SNS has been used as a treatment for FI for twenty years, and it is believed to improve FI. It stimulates sacral nerves and associated muscles by applying a low-voltage electrical current through the sacral foramen via an implanted electrode. The surgical placement of a tined lead with electrodes through the S3 sacral foramen is required. The lead is attached to the pulse generator's battery and placed beneath the lumbar region of the patient's skin (Fig. 3). Thus, SNS is commonly referred to as a minimally invasive treatment for FI [38]. Recently, SNS devices that are rechargeable and MRI-safe have emerged. The estimated battery lifespan of rechargeable devices was 15 years, whereas the estimated battery lifespan of charge-free devices was 5 to 7 years. In addition, these new devices offer advantages to patients who require an MRI [39]. The benefit of SNS is that there is no incision around the anus. This prevents scarring and infection near the anus. Numerous studies have been conducted on SNS.
The benefit of SNS is that there is no incision around the anus. This prevents scarring and infection near the anus. According to some reports, SNS is effective at enhancing FI. In multiple cross-over studies, the incidence of FI was reduced relative to medical therapy [40].
Percutaneous stimulation of the tibial nerve is an additional nerve modulation treatment for FI. The tibial nerve and sacral nerve share nerve fibers. According to some studies, percutaneous tibial nerve stimulation yielded comparable results to SNS [41].
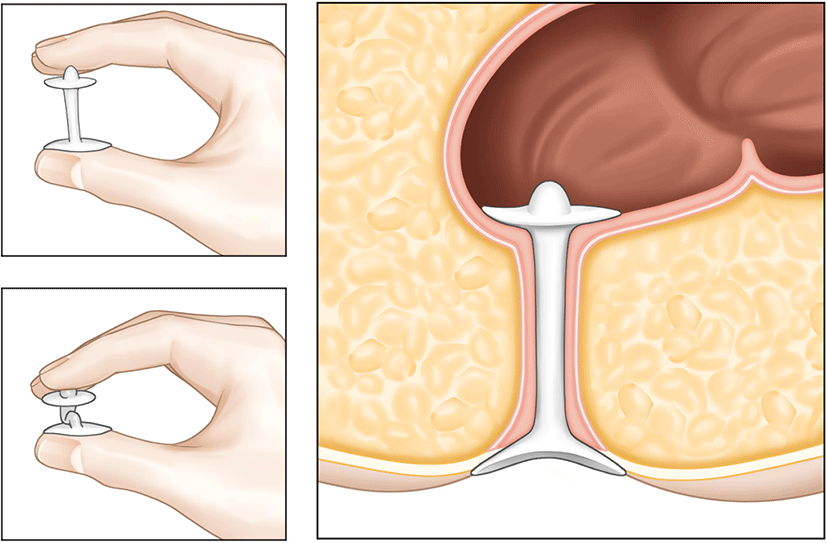
Anal plugs are used to prevent stool leakage by occluding the anus. The high failure rate of anal plugs limits their use as a treatment. However, it is believed to be beneficial for immobile patients or those residing in nursing homes with diminished anal-rectal sensation. The Renew Insert anal plug is designed for self-insertion and natural expulsion with defecation (Fig. 4). Among 91 patients treated with the Renew insert, 73 (80%) completed all 12 weeks of treatment ("completers"), while 85 (93%) completed at least 1 week of treatment (modified intention-to-treat cohort). 77% of the 73 participants who completed the study experienced a 50% reduction in incontinence frequency. 51% of patients experienced complications, but 98% of these were minor, including anorectal urgency, irritation, GI discomfort, gas, and hemorrhoids. Additionally, 78% of participants were pleased with the device [42].
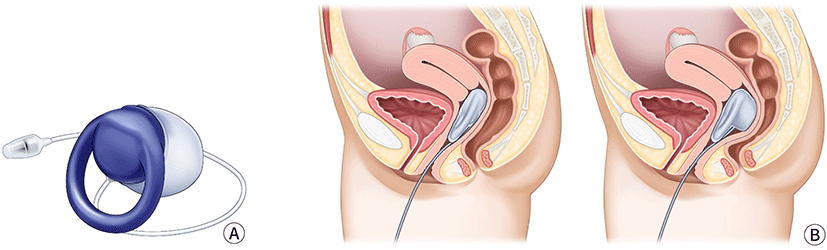
A vaginal bowel control system (Eclipse System) was designed to offer women with FI a low-risk, reversible treatment (Fig. 5). The system consisted of a silicone-coated base, an inflatable balloon, and a hand-held pressure control pump. This device is inserted vaginally. When the balloon is inflated, the vaginal wall is compressed to prevent stool leakage.
Perianal injectables increase the resting anal sphincter tone by acting as a bulking agent. Its primary use is for the treatment of minor FI caused by dysfunction of the internal anal sphincter. Bulking materials injected into the submucosa or inter-sphincteric space increase tissue volume in the high-pressure zone, resulting in a tighter anus at rest, and can target defective areas of the internal anal sphincter to create anal canal continuity, if present [34]. The most research has been conducted on non-animal stabilized hyaluronic acid/dextranomer for the treatment of FI resistant to conservative therapy. The procedures were performed while the patient was in the lithotomy, left-lateral, or prone position. Typically, 1 mL of agent is injected through an endoscope into the deep submucosa at four sites (typically 3, 6, 9, and 12 o'clock). Active inflammatory bowel disease, previous anorectal radiation, full-thickness rectal prolapse, and anorectal malformations are contraindications. Post-injection complications consisted primarily of transient bleeding, pain, and discomfort that resolved on their own [43]. Due to concerns regarding the bulking agent's absorption and migration, additional long-term data are still required [34].
In 1999, the SECCA® procedure, which involves the application of temperature-controlled radiofrequency energy to the anal canal, was first used in Mexico to treat FI.
As a result of the thermal energy delivered, there is an immediate contraction of collagen fibers, which is followed by permanent shortening via remodeling, resulting in a tightening of the anal sphincter muscle. In the SECCA® procedure, radiofrequency is delivered to the anal sphincter while monitoring temperature and tissue impedance and cooling the probes at the surface to prevent mucosal damage. If the tissue temperature rises above 85°C at the electrode tip or 42°C at the anoderm surface, the current is automatically cut off (Fig. 6).
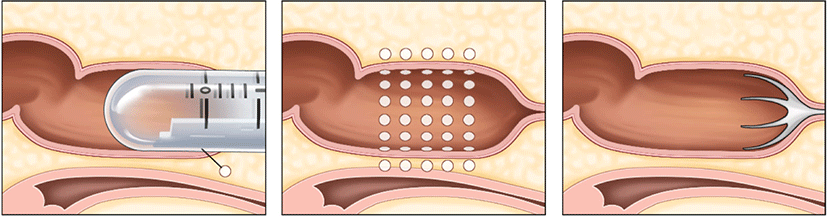
Patients who failed conventional treatments such food modification, medication, and biofeedback and do not have a clearly visible sphincter dysfunction would be indication for SECCA® procedure. Typically, the Secca® operation is carried out as an outpatient while being sedated intravenously and given local anesthesia. T The patient is in the prone jackknife or lithotomy posture. Electrodes are positioned at the level of the dentate line and the device is introduced into the anal canal. The radiofrequency is then transmitted to one quadrant for 90 seconds. The procedure is then repeated for each of the four quadrants at a depth of 5 mm. At the site of insertion, complications include bleeding and ulceration. Occasionally, stitches or surgical therapy may be necessary [44].
Typically, the synthetic sphincter was utilized to treat urine incontinence. The instrument has been adapted for use around the anus (Fig. 7). To attach an artificial anus to the patient's body, a transverse perineal incision is typically made to create a subcutaneous tunnel around the anus. The prosthetic sphincter cuff is positioned around the anus. The pump is inserted via a pfannensteil incision that extends to the labia or scrotum. The reservoir is located in the Retzius space. The gadget provides continence by maintaining a full cuff in a resting condition. When a patient needs to defecate, fluid is pumped from the cuff to the reservoir. And the cuff will passively refill. This procedure must require a sufficient quantity of perineal soft tissue. Because the device is not covered by thin soft tissue, erosion can occur. It is also necessary to instruct patients to activate the device independently. Magnetic anal sphincter is a new artificial anal sphincter option. A single perineal incision is used in the procedure. The perineal body was dissected to a depth of 5 cm. The left and right fossa ischiorectalis were then tunneled, with the tip of the coccyx as a landmark. After the tunneling was completed, the sizing tool was used to determine the proper sized device. Fluoroscopy was used to confirm the correct placement and contact of the beads. For the treatment of severe FI patients, magnetic anal sphincter has shown consistent results [45].
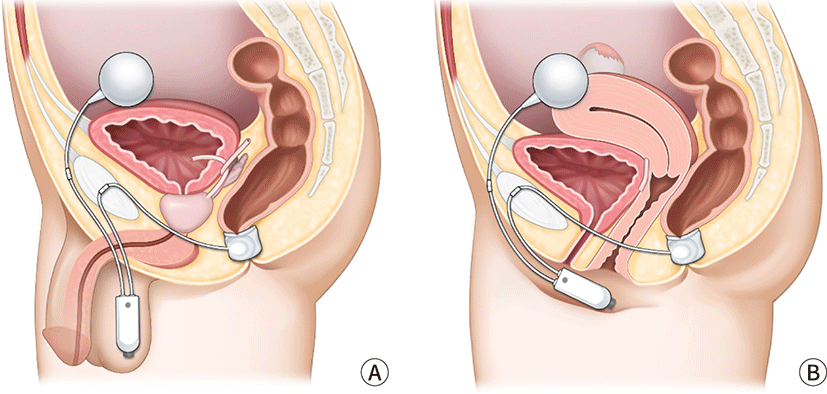
Postoperative complications were discovered in a multicenter study [46]. There were 384 device-related complications in 99 of the 112 patients who received artificial sphincter treatment. 246 events did not necessitate intervention. However, 73 revisional operations were performed on 51 (46%) of the patients. 28 (25%) patients developed infections that necessitated surgical revision, and 41 (37%) patients had their devices completely removed.
In 2008, stem cell therapies were validated. In clinical and experimental contexts involving hematological, cardiovascular, neurological, digestive, traumatic, endocrine, renal, and metabolic disorders, stem cell therapy has been shown to be safe and to have encouraging effects. Hematopoietic stem cells, mesenchymal stem cells, and adipose-derived stem cells are utilized most frequently. It has also been demonstrated that stem cell therapy promotes the healing of acute and subacute anal sphincter injuries. On bioengineered scaffolds, the formation of an innervated anal sphincter was accomplished by combining stem cells with normal cells. According to some reports, stem cell therapy is a successful treatment for FI; nevertheless, additional research in both animal models and clinical settings is required to determine its efficacy. Nonetheless, stem cell therapy is one of the potential treatments for FI [47].
Surgical therapy is also beneficial for treating FI symptoms. Because surgery is an intrusive treatment, many practitioners should evaluate the co-morbidities, socioeconomic circumstances, and activity levels of their patients [48].
Sphincteroplasty aims to restore sphincter architecture and function. Although anal sphincteroplasty gives temporary symptom relief, it rarely results in permanent continence recovery. Surgical consequences consist of wound dehiscence, nerve injury, and infection, and older patients have poorer outcomes. The most severe side effect of sphincteroplasty is rectovaginal fistula. For the treatment of obstetric FI, sphincteroplasty has favorable short- to medium-term outcomes in terms of continence and quality of life [7,49].
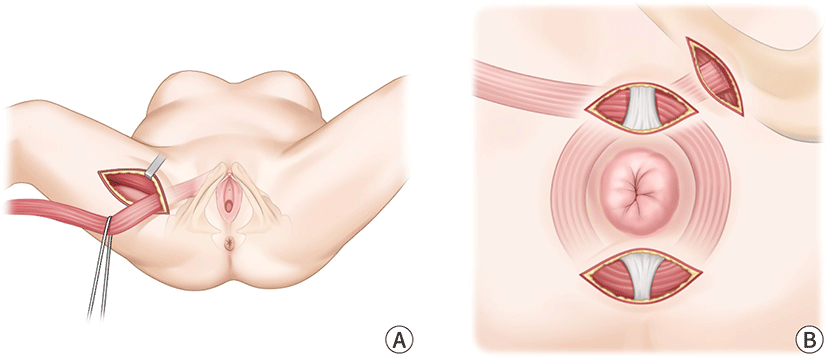
The transposition of the gracilics muscle is an another method that can be used during surgery (Fig. 8). Patients who did not respond to conventional medical treatment for FI may be candidates for this technique. However, there is a considerable risk of problems occurring over the course of this surgery. Sexual dysfunction and soreness in the area of the operation site are also common negative outcomes associated with this procedure [1].
Transposition of the antropyloric valve has recently emerged as a therapy for FI. Beginning the process with a midline laparotomy incision. The colonic hepatic flexure was mobilized, and the duodenum was kocherized. The gastro epiploic branches and larger stomach branch curvature were ligated. The left gastroepiploic pedicle was then utilized to mobilize the antropyloric valve. The pelvic dissection was followed by rectum mobilization and incision. The portion of the antropyloric valve was sutured to the proximal rectal end. Invagination of the graft into the distal rectum. The end of the duodenum was sutured using perianal skin. Transposition of the antropyloric valve perineally is a unique procedure for individuals with significantly compromised anorectal function. Complications of gastrojejunostomy, such as dumping syndrome and alkaline reflux syndrome, were common with this treatment [50]. In patients who did not respond to the aforementioned treatment and had a severely diminished quality of life, the creation of an ostomy would be considered.
Conclusion
With the advent of medicine, patients' lifespans have been lengthened. Consequently, numerous studies on diseases with a high prevalence among the elderly have been done. Incontinence of feces is a prevalent condition in the older population. Patients' quality of life is negatively impacted by this disease, thus it is evidently a disease that requires control. It has not been demonstrated which treatment option for FI is the most effective (Table 3). Nevertheless, the majority of therapy techniques often progress from medicinal treatment to a more intrusive strategy. In addition, when applied clinically to a patient, multiple treatments are administered concurrently, as opposed to a single treatment. Examples include SNS, stem cell therapy, and BFT. It indicates that there is no recognized way for treating FI, but that these various treatment strategies have a small impact on the improvement of patients' symptoms. It is believed that research on a definitive therapy for FI will be required in the foreseeable future. This is important in order to be prepared for an increase in the number of patients.

