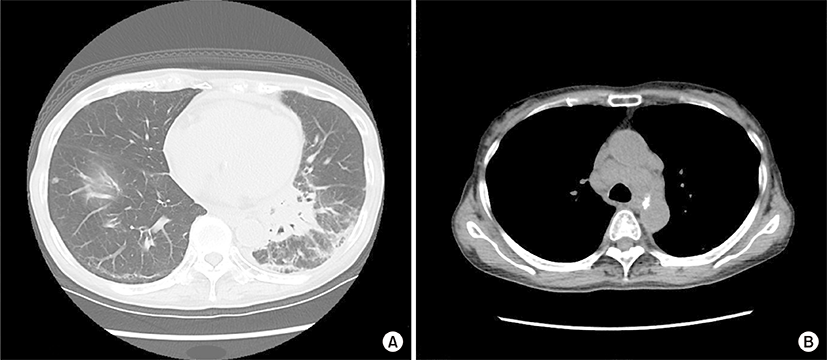
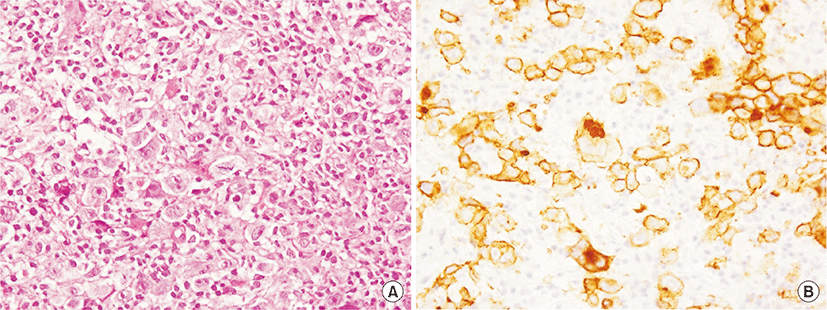
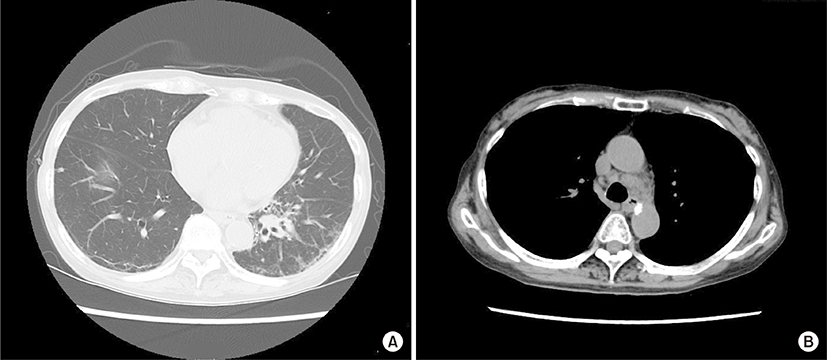
An 84-year-old woman was admitted due to a 2-week history of cough and fever. At 65 years of age, she was diagnosed with primary biliary cirrhosis (PBC) and was successfully treated with ursodeoxycholic acid (600 mg/day) since then. At 81 years of age, she had visited our hospital because of Raynaud’s phenomenon and arthralgia. Her laboratory findings at that time were as follows: white blood cell count, 7,340/μL; (neutrophils, 66.5%; basophils, 0.3%; eosinophils, 4.1%; lymphocytes, 23.6%; monocytes, 5.5%); aspartate aminotransferase, 29 U/L; alanine aminotransferase, 34 U/L; alkaline phosphatase, 303 U/L; gamma glutamyl transferase, 46 U/L; lactate dehydrogenase (LDH), 253 U/L (normal range, 115 to 245 U/L); C-reactive protein, 0.20 mg/dL; immunoglobulin G (IgG), 1,661 mg/dL (normal range, 870 to 1,700 mg/dL); IgM, 595 mg/dL (normal range, 35 to 220 mg/dL); IgA, 402 mg/dL (normal range, 110 to 410 mg/dL); C3, 115 mg/dL (normal range, 80 to 140 mg/dL); C4, 29 mg/dL (normal range, 17 to 45 mg/dL); and rheumatoid factor, 34 IU/L (normal value, <15.0 IU/L). Cryoglobulinemia was not detected. The anti-nuclear antibody titer was 1:640 with a speckled pattern, and anti-SS-A and anti-mitochondrial antibodies were positive. Negative results were obtained for anti-SS-B and anti-cyclic citrullinated peptide antibodies. Chest roentgenogram and chest computed tomography (CT) revealed interstitial opacities, predominantly in the bilateral lower lung fields. On the basis of these laboratory findings, and positive results for Shirmer test, Rose Bengal test, and lip biopsy, the patient was diagnosed with primary Sjögren’s syndrome (SS) accompanied by interstitial pneumonitis and PBC. Eight months before this episode of cough and fever, chest roentgenogram had remained unchanged but LDH levels had increased to 341 U/L. At this admission, physical examination revealed neither superficial lymph nodes nor swollen salivary glands. The IgG, IgM, IgA, and LDH levels were 1,284 mg/dL, 261 mg/mL, 309 mg/dL, and 616 U/L, respectively. Chest CT revealed a mass shadow involving the bronchus along with interstitial and consolidation opacities in the left lower lung field (Fig. 1A) as well as enlarged bilateral hilar and mediastinal lymph nodes (Fig. 1B). However, enlarged cervical, axillary, and para-aortic lymph nodes were not detected on CT examination. Transbronchial lung biopsy specimens revealed scattered large atypical cells, resembling Hodgkin and Reed-Sternberg cells in the background of lymphocytes (Fig. 2A). The large atypical cells were positive for CD30 (Fig. 2B) and CD15 and negative for CD3 and CD20, as determined by immunohistochemical staining. These cells were negative for Epstein-Barr virus (EBV) on in situ hybridization. Finally, the patient was diagnosed with primary pulmonary Hodgkin lymphoma (HL) associated with primary SS. The patient refused to receive conventional chemotherapy or radiotherapy. She was treated with an alternative therapy of prednisolone (20 mg/day, for the first 14 days; 15 mg/day for the next 14 days; and 10 mg/day thereafter) in combination with cyclophosphamide (50 mg/day) and clarithromycin (800 mg/day), a macrolide, which is known to have anti-lymphoproliferative effects [1,2]. Ten weeks after treatment initiation, the aforementioned symptoms as well as abnormal findings on chest CT showed considerable improvements (Fig. 3).
Lymphoma was first described in patients with SS in 1951 [3]. The relative risk of developing lymphoma was about 16-fold higher in SS patients than in the general population [4]. Most lymphomas associated with SS are extranodal marginal zone lymphomas, followed by diffuse large B-cell lymphomas [4]. Only few cases of HL associated with SS have been reported [5-9]. According to these previous reports and the present case report, all patients were women, unlike those with HL not associated with SS. In previous case reports, the onset of lymphomas was indicated by parotid gland swelling and/or palpable lymphadenopathy [5-9]. In the present case, the presence of abnormal shadows on chest CT images alone led to the diagnosis of HL. Pulmonary involvement has been reported in 15%–40% of patients with HL. However, the presentation of primary pulmonary HL is very rare, with just over 70 cases reported worldwide [10]. To the best of our knowledge, the present case is the first report of a primary pulmonary HL associated with primary SS.
EBV is considered to be one of the etiologic factors associated with HD. In fact, Navarro et al. [5] identified the presence of EBV markers in the tumor cells of patients diagnosed with primary SS who subsequently developed HL. However, in the present case, EBV was not detected in the tumor cells. A possible mechanism of lymphoma development might be preceding pulmonary inflammation related to interstitial pneumonitis.
At SS diagnosis, the laboratory and clinical features such as low C4 levels (<12 mg/dL), cryoglobulinemia, neutropenia, lymphopenia (<1,000/μL), purpura, and splenomegaly are identified as major non-HL predictors [11]. In the present case, none of the aforementioned features were recognized. During clinical course, laboratory predictors of lymphoma development are known to show a fall in rheumatic factor and gamma globulin levels. Vivancos et al. [6] reported a fall in serum gamma globulin levels. However, Lima et al. [7] reported a persistent hypergammaglobulinemia. In the present case, although a fall in gamma globulin levels was detected at the onset of lymphoma, a rapid increase in LDH was prominent, which might be associated with HL as well as interstitial pneumonitis.
Nevertheless, considering the low incidence of HL associated with SS, patients with SS who develop HL must be closely monitored to better elucidate the etiology, predictors, and the prognosis of the condition.