Introduction
Invasive lobular carcinoma (ILC) is the second most common subtype of invasive breast cancer after invasive ductal carcinoma (IDC). ILC represents 5% to 15% of all cases of newly diagnosed breast cancers [1-3]. The biological and clinical features of ILC differ from those of IDC. Compared with IDC, ILC shows estrogen/progesterone receptor positivity, HER2/neu negativity, bcl-2 positivity, p53 and vascular endothelial growth factor negativity, low grade, low likelihood of lymphovascular invasion and comparable or slightly lower lymph node involvement [2,4]. ILC occurs more frequently in older patients and presents with larger tumor size than IDC [2]. ILC tends to be multifocal, multicentric and/or bilateral cancers and more frequently metastasizes to the peritoneum/retroperitoneum, ovary and gastrointestinal tract [2,5]. Therefore, ILC diagnosed and treated more cautiously compared with IDC.
Histopathologically, ILC is characterized by a diffusely infiltrative growth pattern and very little desmoplastic stromal reaction. These features reflect the typical loss of the adhesion molecule, E-cadherin [6,7]. Thus, ILC is difficult to detect clinically, radiologically and even pathologically [8-10]. The ILC detection sensitivities of mammography and ultrasound (US) have been reported as low as 57% to 81% [8,11] and 68% to 98% [12,13], respectively. Magnetic resonance imaging (MRI) has an overall sensitivity of 93.3% for detection of ILC and is the imaging modality of choice. MRI detects additional ipsilateral lesions in about one-third of ILC patients and contralateral lesions in 7% of patients [14]. Recent studies have demonstrated the diagnostic performance of emerging imaging modalities for the detection of ILC [15-19]. Breast specific gamma imaging (BSGI) shows the highest sensitivity compared to mammography, US and MRI in the detection of ILC [17]. By comparison, fludeoxyglucose F18 (18F-FDG) positron emission tomography/computed tomography (PET/CT) has a lower detection rate for primary ILC and lower sensitivity for additional ipsilateral lesions than MRI [18]. Digital breast tomosynthesis (DBT) is an emerging technology that has been shown to greatly reduce normal overlapping breast parenchymal tissue and improve the detection of breast cancer [20-22]. DBT plus two-dimensional (2D) mammography can detect more breast cancers than 2D mammography alone [16]. DBT plus digital mammography (DM) reportedly significantly improves the diagnostic accuracy of ILC than DM alone [19]. However, detection of ILC using DBT remains in need of comprehensive investigation.
Presently we compared the diagnostic performances of DM, US, BSGI, PET/CT, DBT, and MRI for the detection of ILC, including both index cancers and multifocal/multicentric (multiple) suspicious lesions.
Methods
This retrospective study was conducted with institutional review board approval (2017-02-037). Patient informed consent was waived since all patient records/information were anonymized and de-identified prior to analysis. Between October 2011 and November 2015, 1,673 patients underwent breast surgeries due to breast cancers in our institution. Of these, 78 breasts in 76 women (mean age, 51 years; range, 33 to 85 years) had surgically proven ILCs and were enrolled in this study. Demographic characteristics of patients are presented in Table 1. ILCs were analyzed per breast. Prior to the surgery, all patients received imaging modalities including DM, DBT, US, MRI, BSGI, and/or PET/CT. All patients received at least one imaging modality for preoperative staging. Among multiple imaging modalities, preoperative imaging modalities for ILCs were performed, under the necessity of surgeons and radiologists.
Two views (craniocaudal and mediolateral oblique) of DM and/or DBT of each breast were performed using a commercially available system (Dimension; Hologic, Bedford, MA, USA). The device consisted of a custom designed high-power (mA) tungsten (W) anode X-ray tube with rhodium, silver and aluminum X-ray filters. Different filters used in the DM and DBT imaging modes produced optimal X-ray spectra based on the breast thickness/composition of the breast using automatic exposure control. The X-ray tube moved over a 15o arc while the breast was compressed, taking a series of ultra-low dose mammographies. The mean grandular doses of DM and DBT were 1.2 and 1.5 mGy, respectively. DM was performed for all patients with ILCs. If the patient underwent DM at other hospital, additional DBT was performed. If architectural distortion was detected on DM, additional DBT was performed.
Bilateral whole breast hand-held US examinations were performed by one of the four board-certified radiologists (ESC, JEL, JC, and JHK) with 6 to 26 years of breast imaging. US images were obtained with one of the commercially available iu 22 scanning devices (Philips Medical Systems, Bothell, WA, USA), the Aixplorer system (Supersonic Imagine, Aix en Provence, France) or the GE LOGIQ9 (GE Medical Systems, Milwaukee, WI, USA) with a 7.5- to 15-MHz linear array transducer. At least two orthogonal views (transverse/longitudinal and/or radial/antiradial) were obtained per lesion. Color Doppler and/or elastography were used based on the radiologist’s decision.
Breast MRI was performed with an Achieva 3-T system (Philips Healthcare, Amsterdam, Netherlands) and a dedicated Sense Breast 7 coil. Breast MRI was done with the patients in a prone position. Before injection of contrast medium, bilateral axial fat suppressed T2 weighted images were obtained. Dynamic contrast-enhanced T1-weighted sequences with high resolution isotropic volume excitation (Achieva) were obtained after bolus injection of 0.1 mL/kg of gadolinium-diethylenetriamine penta-acetic acid contrast medium (Gadovist; Bayer Schering Pharma AG, Berlin, Germany) and followed by a 25-mL saline flushing through automatic injector at a rate of 2 mL/sec. Both breasts were scanned in the axial plane and the lesion containing breast was additionally scanned in the sagittal plane. After intravenous injection of contrast medium, six phases of dynamic images were obtained at 55.4 seconds (axial), 110.8 seconds (axial), 146 seconds (sagittal), 221.6 seconds (axial), 292 seconds (sagittal), and 438 seconds (axial). Standard subtraction images were made from the non-enhanced and early and late enhanced FLASH (fast low angle shot) sequences. Multiplanar reconstruction and maximum-intensity-projection reconstruction images with coronal and sagittal planes were also acquired.
Some patients underwent BSGI to evaluate local tumor extent after diagnostic confirmation of malignancy. BSGI studies were performed at least 2 weeks after biopsy to prevent potential confounding effects [23]. BSGI was performed with patients in the sitting position and with a dedicated high-resolution breast-specific gamma camera (Dilon 6800; Dilon Technologies, Newport News, VA, USA) after intravenous injection of 555 to 925 MBq of 99mTc-methoxyisobutylisonitrile. After 10 minutes, planar images were obtained in the craniocaudal and mediolateral oblique projections of both breasts.
18F-FDG PET/CT was performed for preoperative staging work-up after diagnostic confirmation of malignancy. PET/CT studies were performed also at least 2 weeks after core needle biopsy [23]. PET/CT was scanned from skull base to thigh with the patient in the supine position using a Biograph mCT dedicated whole body PET/CT scanner (Simens Biograph mCT with 128 slice CT; Simens Medical Solutions, Knoxvile, TN, USA). The patients fasted for at least 6 hours and serum glucose levels were below 140 mg/dL. One hour after the intravenous administration of 5.18 MBq/kg of FDG, low-dose CT (120 kVp/50 mAs) without contrast enhancement was acquired. The PET images were reconstructed using iterative reconstruction algorithm and attenuation correction with combined CT scan.
One of four board-certified breast radiologists randomly and independently interpreted all images including DM, DBT, US, and MRI. According to the ACR BI-RADS lexicon, final assessment was recorded in the radiologic reports of the images. BSGI and PET/CT images were interpreted by one of two board-certified nuclear medicine specialists and were averaged.
The detection of ILC on various imaging modalities were classified as positive or negative detection for each index cancer per breast. Multiple ILCs were also analyzed using various imaging modalities. Positive detection was defined as BI-RADS category 4 and 5 lesions for DM, DBT, US, and MRI, while focal increased radiotracer or FDG uptake was regarded as positive detection for BSGI and PET/CT. Negative detection was defined as BI-RADS category 1 to 3 lesions for DM, DBT, US and MRI. For BSGI and PET/CT, negative detection was defined as no focal increased radiotracer or FDG uptake or scattered heterogeneous physiologic uptake. The results of imaging finding, which were not retrospectively reviewed for this study, based on the original radiological reports. The final surgical pathologic report was the reference standard.
The detection rates of DM, DBT, US, MRI, BSGI, and PET/CT for ILC were calculated for each index cancer per breast. Pathologically proven multifocal/multicentric lesions were defined as multiple ILCs. Diagnostic performances of various imaging modalities for detection of multifocal/multicentric (multiple) suspicious lesions were assessed as follows: sensitivity, specificity, positive predictive value, negative predictive value (NPV), and accuracy. True positive indicates that patients with suspicious multifocal/multicentric lesions on images with pathologically proven multifocal/multicentric lesions. False positive indicates that patients with suspicious multifocal/multicentric lesions on images with pathologically proven as index ILC. False negative indicates that patients with no suspicious multifocal/multicentric lesions on images, though pathologically proven as multifocal/multicentric cancers. True negative lesions indicates that patients with pathologically proven as index ILC without suspicion on images. We used either the chi-square test or the Fisher exact test for comparison of diagnostic performances between imaging modalities. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA).
Results
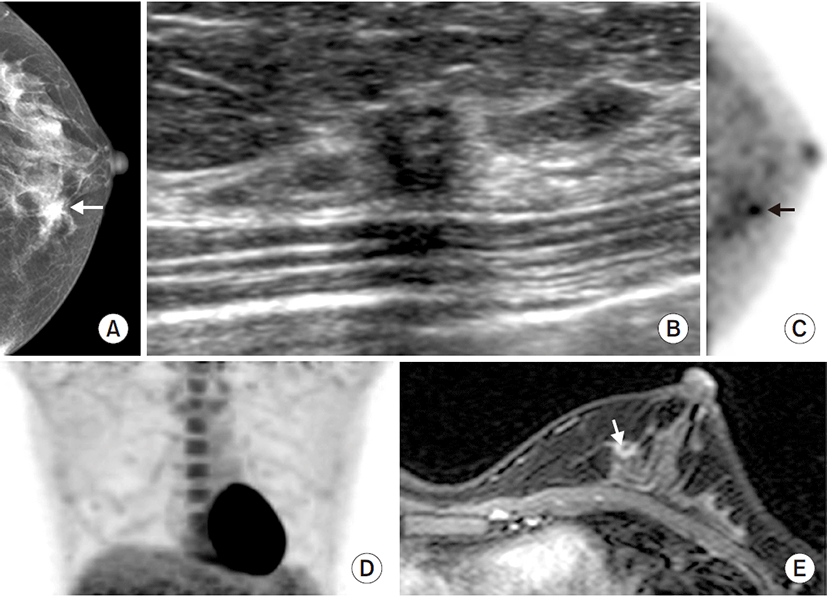
Seventy-eight biopsy proven index ILCs were diagnosed in the 76 women and two patients had bilateral ILCs. ILCs were analyzed per breast. The mean pathologic size of the index cancer was 26.6 mm (range, 4 to 118 mm). Mastectomy was performed in 26 breasts (33.3%) and conserving surgery was performed in 52 breasts (66.7%). Pathologic proven multiple ILCs were diagnosed in 26 breasts (33.3%). Prior to surgery, patients underwent DM (n=72), DBT (n=15), US (n=77), MRI (n=76), BSGI (n=50), and PET/CT (n=74). Table 2 summarizes the detection rates of each imaging modality for index cancer. The detection rates of DBT, US, and MRI for index cancers were 100%. The detection rates of BSGI and PET/CT were 96% (48/50) and 93.2% (69/74), respectively. The detection rate of DM for index cancer was 87.5% (63/72). DBT, US, and MRI showed the highest detection rate for index cancer, followed by BSGI, PET, and DM (Fig. 1).
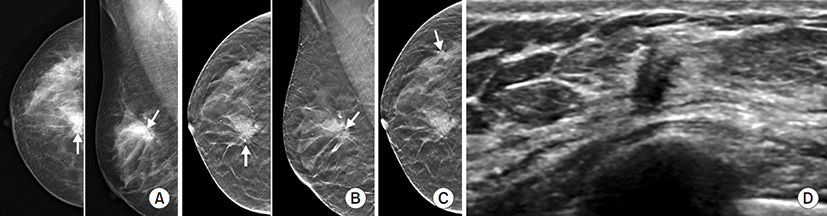
Table 3 summarizes the data concerning multiple suspicious lesions on various imaging modalities and pathologically proven multiple ILCs. Additional suspicious lesions were detected in 14 cases on DM, 12 cases on DBT, 38 cases on US, 51 cases on MRI, 15 cases on BSGI, and 23 cases on PET/CT. Multiple suspicious lesions at any imaging modality were detected in 57 breasts. Among them, 51 lesions were detected on MRI and/or other imaging modalities. Five lesions were detected on US and/or other imaging modalities (DM, BSGI, and PET/CT). One lesion was detected by both BSGI and PET/CT. Pathologic correlation with localization was performed in 13 cases. Among them, nine cases were surgically proven ILCs. The remaining 44 cases underwent surgeries (27 breast conserving surgeries and 17 mastectomies) without localization. Seven ILCs were found after breast conserving surgeries and 10 ILCs were additionally confirmed on mastectomy specimens. True-positive multiple ILCs were detected 42.9% (6/14) on DM, 66.7% (8/12) on DBT, 55.3% (21/38) on US, 51% (26/51) on MRI, 46.7% (7/15) on BSGI, and 60.9% (14/23) on PET/CT (Table 3). Pathologically finally-proven multiple ILCs of each modality were 25 on DM, eight on DBT, 26 on US and MRI, 20 on BSGI, and 25 on PET/CT (Table 3).
The diagnostic performance of each imaging modality for multiple ILCs is presented in Table 4. DBT and MRI had 100% sensitivities for multiple ILCs (Fig. 2), followed by US (80.8%), PET/CT (56%), BSGI (35%), and DM (24%). The sensitivities of multiple ILCs were significantly different between each modality (P<0.001). DBT (73.3%) and PET/CT (73.0%) had higher accuracy, and DM (62.5%) and BSGI (58%) had lower accuracy, for multiple ILCs. However, there was no statistical significance of diagnostic accuracy between each imaging modality (P=0.460). Unlike BSGI, which showed the lowest NPV (62.9%), DBT and MRI had a NPV of 100%. NPVs were significantly different between each modality (P=0.001). The specificity of DM (83.0%) was the best and of DBT (42.9%) was the worst among various modalities (P=0.002). The positive predictive value of DBT was the best (66.7%), although not significant (P=0.786).
Discussion
This study compared the diagnostic performance of various breast imaging modalities for ILC, both index cancer and multiple ILCs. Among various imaging modalities, DBT, US and MRI showed excellent detection rate of 100% for index cancer. For multiple ILCs, PET/CT, and DBT had higher accuracy. Especially, DBT had a sensitivity of 100% and a NPV of 100%.
While DBT showed a perfect diagnostic performance for both index and multiple ILC, DM showed the lowest detection rate for index ILC, and the lowest sensitivity and accuracy for multiple ILCs. Mammography is the main screening tool for early detection of breast cancer. However, detection of ILC on mammography is challenging. Berg et al. [24] reported sensitivities of mammography of 81% for IDC and 34% for ILC. However, sensitivities for patients with dense breasts were markedly decreased to 60% for IDC and 11% for ILC. The most common mammographic finding of ILC is spiculated, ill-defined mass/asymmetry without central increased density [9,25]. The similar density between ILC and surrounding breast tissue makes it difficult to diagnose ILC on mammography. ILC also commonly presents as an architectural distortion [9,25]. Architectural distortion is an established suspicious mammographic finding that can often be inconspicuous. DBT minimizes the influence of normal overlapping or superimposed breast tissue [16]. Especially, DBT has a unique strength for architectural distortion/asymmetries and for dense breasts [26,27]. Therefore, DBT may have an advantage over conventional 2D mammography in the detection of ILC [15]. In one study, DBT plus DM increased the detection rate for ILC from 0.27 to 0.55 per 1,000 cases compared with DM alone [16]. In a recent multi-reader study for ILC, DM plus DBT had significantly higher area under the curve and sensitivity than DM alone [19]. The improvement of interpretive performance was prominent for less-experienced radiologists. Multiple and bilateral lesions was also more frequently detected on DM plus DBT. Our results also support that DBT is superior to DM for the diagnosis of ILC.
Presently, DBT showed the second highest diagnostic accuracy of 73.3% for multiple ILCs after PET/CT (73.6%). However, there was no statistical significance. Meanwhile, specificity for multiple ILCs was higher in DM (82.9%) than in DBT (42.9%) (P=0.039). MRI showed significantly higher sensitivity and specificity than PET/CT for multiple ILCs. ILC most commonly presents as an irregular or spiculated mass on MRI, followed by non-mass lesion [14]. When ILC presents as non-mass lesion, it may show variable distributions of ductal, segmental, regional or diffuse patterns [14]. The enhancement kinetics of ILC differs a little from that of IDC. Compared with IDC, ILC more slowly attains to peak enhancement. The proportion of ILC that features delayed phase washout is smaller than that of IDC [14]. Meanwhile, ILC shows lower 18F-FDG uptake than IDC on PET/CT, which was due to its unique diffusely infiltrative growth pattern, lower tumor cellularity, lower glucose transporter 1 expression and lower proliferation rate [28]. The effectiveness of MRI or PET/CT in diagnosing multiplicity of ILCs has been documented [14,18]. In a meta-analysis of studies using MRI in patients with ILC, MRI detected additional ipsilateral lesions in 32% of patients [14]. A recent study compared the diagnostic performance between MRI and PET/CT in patients with ILC [18]. In the study, MRI had significantly higher sensitivity and lower specificity than PET/CT for multiple ILCs. Diagnostic accuracy of MRI and PET/CT were not significantly different (65.6% vs. 68.8%). In our study, MRI detected multiple ILCs in 37% (28/51) of patients who underwent MRI and 23% (17/23) of patients who underwent PET/CT. MRI had significantly higher sensitivity (P=0.002) and higher specificity (P=0.001) than PET/CT for multiple ILCs. Overall accuracy of PET/CT for multiple ILCs was the best among the six different breast imaging modalities, although this result did not attain significance. Thus, PET/CT is a complementary tool in combination with MRI in the preoperative assessment of ILC, especially for detection of multiplicity.
In a previous study [17], BSGI showed the greatest sensitivity for detecting ILC followed by MRI, mammography, and US. On the other hand, in the present study, BSGI had a detection rate of 96% and DBT, US and MRI had a detection rate of 100% for index ILC. Similarly in multiple ILC, BSGI showed relatively low diagnostic performances with DM among various imaging modalities. In comparison with MRI, BSGI had significantly lower sensitivity (P<0.001), lower specificity (P=0.001) and lower negative predictive value (P=0.015). BSGI, as a functional imaging tool, has comparable sensitivity and greater specificity than MRI for breast cancer [29,30]. Our results for index ILC were consistent with previous studies. However, BSGI declined diagnostic power for multiple ILC than MRI. The prior superior results of BSGI were limited to the index cancer [29,30]. Additional detected multiple ILCs were usually smaller than index cancer. The intrinsic size resolution due to functional image may be the main drawback to detection of multiple ILCs.
Our study had some limitations. First, this study was a retrospective-designed, single-center study. Second, all patients did not receive all six different imaging modalities. Especially, DBT was performed for only 19.7% (15/76) of all patients. Thus, further prospective studies that include a large cohort of ILC with DBT are needed. Third, localization of multiple suspicious lesions was not complete. Of 57 multiple suspicious lesions, 44 were treated by surgery (17 mastectomies and 27 conserving surgeries) without localization and 17 multiple ILCs was confirmed on surgical specimens. Fourth, 52 of 78 breasts with ILC underwent breast conserving surgery. Therefore, additional ipsilateral or contralateral malignancies may be underestimated if the lesion was not detected in any preoperative imaging evaluation. Fifth, acquired position of each imaging modality is different, thus the detection rate of breast cancer may vary from these different positions. Nevertheless, this study is the first to compare the diagnostic performance of various breast imaging modalities including DBT for ILC. Additionally, we evaluate diagnostic performance for both index and multiple ILCs.
In conclusion, DBT, US, and MRI showed 100% detection rate of index ILCs. For multiple ILCs, DBT and PET/CT were accurate imaging modalities, whereas DM and BSGI showed relatively low diagnostic performances. DBT and PET/CT are effective modalities for patients with ILCs and have promising roles in the diagnosis of multiple ILCs.