Introduction
Brugada syndrome (BS) is a rare genetic autosomal dominant disease with incomplete penetrance which affects ion channels of the cardiac conduction system and causes a coved ST segment elevation in the right precordial leads with a pseudo right bundle branch block pattern and susceptibility to ventricular arrhythmias that may cause syncope or sudden death [1]. This disorder raises specific concerns as anesthesiologists routinely administer drugs that interact with cardiac ion channels which could trigger the development of malignant ventricular arrhythmias.
There are few reports of regional anesthetic management of patients with BS, and especially brachial plexus block (BPB) of patients with BS. Therefore, we report successful anesthetic management for a patient with BS for tenorrhaphy under BPB after 8 hours later end of 1st operation under general endotracheal anesthesia in a day.
Case
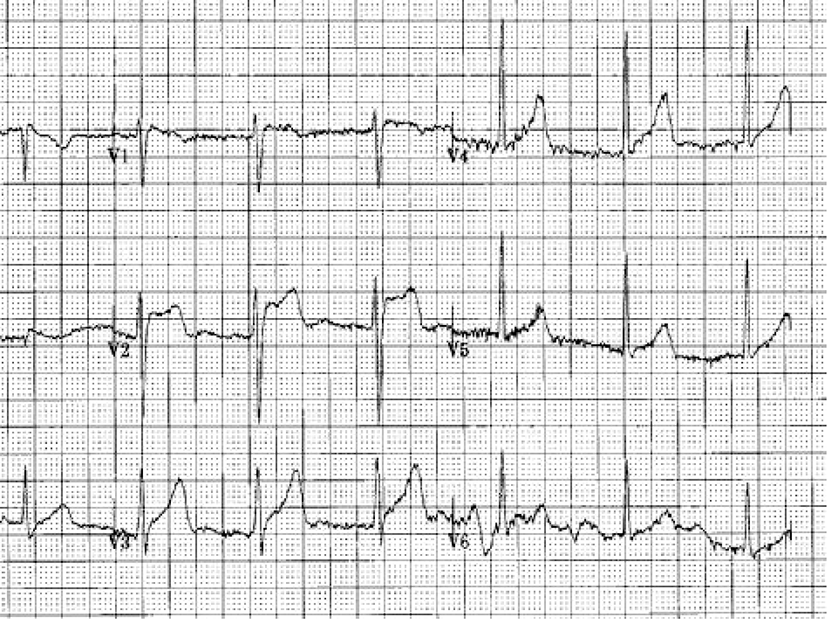
A heavily drunk 30-year-old man was presented for an emergency exploratory laparotomy due to right upper and lower abdominal stab wound at 2 a.m. He had no underlying disease and no specific past history except splenectomy under general anesthesia 8 years ago due to traffic accident. He had no past history of syncope and there was no family history of sudden death. Chest X-ray showed minimal hemo- and pneumo-thorax on the right side, and abdominal and pelvic 3 dimensional computed tomography showed massive intraperitoneal and retroperitoneal hematoma and free air. His hematocrit changed from 42.9% to 28.5% in an hour and heart rate increased from 68 to 100 bpm, but systolic blood pressure maintained around 90 mmHg. Electrocardiography (ECG) findings revealed right bundle branch block, ST segment elevation in the precordial lead (V2), which suggested BS (Fig. 1), but the patient’s vital sign was getting worse and further evaluation was impossible. Having been informed of the associated risks, the patient was taken to the operating room and routine monitors were applied. A radial arterial cannula was inserted under local anesthesia on left radial artery. Prior to the induction of anesthesia, automatic defibrillator pads for cardioversion were attached to his anterior chest. Anesthesia was induced by thiopental sodium 250 mg, rocuronium 50 mg, remifentanil infusion (20 μg/mL) and was maintained with sevoflurane 0.5 to 2.0 vol% and remifentanil 3 to 5 μg/min. After removal of hematoma in abdominal cavity, systolic blood pressure dropped below 60 mmHg, and 100 μg of phenylephrine administered twice. After the ligation of mesenteric artery branch and transfusion, hemodynamic index was stabilized. Hemothorax seemed to be caused by regurgitated blood from abdominal cavity and chest tube was inserted in right chest. At the end of the operation, intravenous patient controlled analgesia using a mixture of sufentanil and ondansetron was started. Surgery lasted 3 and half hours and estimated blood loss was 2,000 mL. The endotracheal tube was removed after confirming complete recovery of consciousness, adequate spontaneous ventilation. Pyridostigmine and glycopyrrolate were administered as a reversal agent. After the operation, the patient was transferred to intensive care unit with the automatic defibrillator pads. His vital signs were stable and postoperative pain was also controlled with intravenous patient controlled analgesia. His cardiac rhythm was uneventful during surgery and at intensive care unit.
Right 4th and 5th finger tendon ruptures were found at intensive care unit and he was transferred to operating room for tenorrhaphy at 4 p.m., 8 hours later end of 1st operation. BPB was done because the patient strongly refused general anesthesia. All the monitoring and settings were same with previous anesthesia and axillary approach was done using nerve stimulator to minimize depression of respiration. The stimulating needle (2-inch, 22-gauge Stimuplex insulated needle; B. Braun, Berlin, Germany) was connected to a nerve stimulator (Stimuplex-DIG Stim-300, B. Braun) at an initial current intensity of 0.5 mA and was advanced until it elicited motor responses in the distribution of the axillary, musculocutaneous, ulna or median nerves. The current was gradually decreased to a range of 0.3 to 0.4 mA, with a persistent acceptable motor response.
In total, 30 mL of the 2% lidocaine, 0.75% ropivacaine, normal saline (1:1:1 mixture) and 1:200,000 of epinephrine mixture was injected in 5 mL aliquots, with frequent aspirations to assess intravascular needle migration. 5 L/min of oxygen was supplied with simple facial mask and 3 to 5 μg/min remifentanil was administered intravenously for reducing abdominal pain due to prior exploratory laparotomy. The vital sign was stable and there was no change of ECG pattern. After the operation, the patient was transferred to the intensive care unit again. His postoperative cardiac rhythm was uneventful on the day following surgery. The patient was transferred to the general ward 2 days after the operation and there was no episode of tachyarrhythmia throughout his hospital stay.
Discussion
BS is an uncommon autosomal dominant genetic disease with incomplete penetrance and variable expressivity stemming from various mutations of ion channels in the cardiac conduction system. Seven genotypes of BS have been characterized, including mutations in cardiac sodium (types 1, 2, 5, and 7), potassium (type 6), and calcium (types 3 and 4) channels [2].
The type 1 Brugada ECG pattern is the most specific pattern for BS. Type 1 is characterized by a coved-type ST segment elevation of at least 2 mm in the right precordial leads associated with a complete or incomplete right bundle branch block followed by a negative T wave. It has been recommended that all patients with a type 1 ECG abnormality even when isolated, should be considered at risk of sudden death [3]. It would seem sensible to treat these patients undergoing anesthesia as having the condition. In this case, the patient showed type 1 ECG pattern.
Perioperative pharmacological and physiological changes may precipitate malignant arrhythmias and cardiac dysfunction in these patients. These patients show large and conflicting variation in response to certain drugs and conditions [4]. An understanding of the modulating agents and conditions is essential for planning of appropriate techniques and crisis avoidance. Autonomic tone, which affects ion channel flux, can contribute to development of tachyarrhythmia. Factors known to affect autonomic tone such as inadequate analgesia, light anesthesia and postural changes should be minimized. Depth of anesthesia should be balanced to minimize these effects, as bradycardia or increased vagal tone as a result of surgical stimulation have also been implicated in the development of Brugada ECG changes [5].
The choice of induction agent is probably not critical. Midazolam, propofol, barbiturates and fentanyl have all been used successfully on many occasions [4]. However, Kloesel et al. [6] reported that some anesthetic agents (propofol, etomidate, lidocaine, and succinylcholine) were noted to have a temporal association to ST segment elevations.
There is no evidence regarding the ideal volatile anesthetic agent in BS. Isoflurane and sevoflurane have been used in air and nitrous oxide without incident [4]. We used barbiturate as an induction agent and used sevoflurane for maintenance of anesthesia.
Beta-adrenergic blockade and alpha-receptor agonists can aggravate the characteristic ST changes by exacerbating the ion current imbalances during the early part of the myocardial action potential [7]. However, Probst et al. [8] reported that phenylephrine injection did not provoke ST segment elevation and ventricular arrhythmias in BS patients. In this case, phenylephrine was administered twice to treat severe hypotension during the surgery and there was no specific ECG change.
Regional anesthesia had been associated with complication in BS because of the theoretical risk of problems when using local anesthetic drugs with sodium channel blocking properties. Lidocaine is a local anesthetic drug with antiarrhytmic properties when given intravenously in small doses, with class IIb recommendation to be preferably avoided as antiarrhytmic drug in patients with BS [9]. Whenever given their action as sodium channel blockers, caution is advised, and the minimal effective dose should be used while ensuring close monitoring of vital signs and ECG at all times [6]. However, there are some reports of uneventful regional or wound infiltration with local anesthetics in BS patients [10-12]. The authors still advise caution when administering local anesthetics in areas where systemic absorption may be rapid. Duque et al. [13] reviewed a 12-year case series of diagnosed BS and classified patients at high risk of BS.
There were 6 cases of epidural anesthesia using ropivacaine without any of specific event. Although no BPB case has been reported, so far absorption and elimination of the ropivacaine in BPB are similar to that of epidural block, and furthermore, the speed of absorption is slower than that of epidural anesthesia. And when guided with ultrasound, we could even be able to reduce the total volume.
In this case, the lesion was 4th and 5th finger and we used axillary approach for BPB. Radial nerve block was unnecessary and we could reduce the total volume of local anesthetics. Besides the patient had right side hemothorax and had recent abdominal surgery, we tried to avoid any respiratory dysfunction induced by interscalene approach. We used only 75 mg of ropivacaine (10 mL of 0.75% ropivacaine) and it does not reach maximum tolerate intravenous dose of 115 mg in human study [14]. Although, our experience of BPB using ropivacaine in patient with BS was limited, we performed a successful anesthetic management in patients with BS. Accordingly, we can speculate that both general endotracheal anesthesia and BPB via axillary approach can be chosen safely in patients with BS, with provision of appropriate preparation and management.