Introduction
The incidence of rectal cancer in Korea has recently increased; it is one of the common types of cancer, and research interest regarding the treatment and outcomes of this condition has increased [1]. The results of rectal cancer treatment were greatly improved after the introduction of neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), which is now the standard treatment for locally advanced rectal cancer [2,3]. Although the oncologic outcomes have improved with nCRT, recent studies have shown that rectal cancer patients with lateral pelvic lymph node (LPLN) metastasis have poorer outcomes; moreover, LPLN is the main site of local recurrence (LR), which occurs even after nCRT [1,45-6].
However, the management of patients with LPLN remains unclear and varies among institutions and physicians. In Western countries, suspicious LPLN metastasis outside the internal iliac chain has been considered as distant metastasis requiring adjuvant chemotherapy [7]; in the East especially in the Japanese group, it is considered as a local disease, and lateral pelvic nodal dissection (LPLND) is performed [89-10]. However, LPLND requires delicate and difficult technique and can cause several morbidities including large volume of blood loss, urinary retention, and sexual dysfunction [4,11,12]. Indeed, whether LPLN metastasis should be considered as local or distant metastasis remains controversial. Therefore, LPLND must be carefully performed in patients who would benefit from this procedure. However, the criteria for selecting patients who will undergo LPLND have not been clearly established especially under the setting of nCRT. Some studies used changes in LPLN size after nCRT, while others used pre-nCRT LPLN size as the criteria for undergoing LPLND. Moreover, the extent of LPLND remains unclear: LPLN sampling vs. LPLND [6,13,14]. The LPLN size criteria also varied, and the method of diagnosing LPLN metastasis remained debatable [6,1314-15].
This review aimed to present the oncological outcomes of rectal cancer patients with metastatic LPLN who underwent nCRT and the impact of LPLND. In addition, various indications for LPLN metastasis treated with nCRT and the diagnostic criteria were reviewed.
Incidence of LPLN Metastasis with/without nCRT
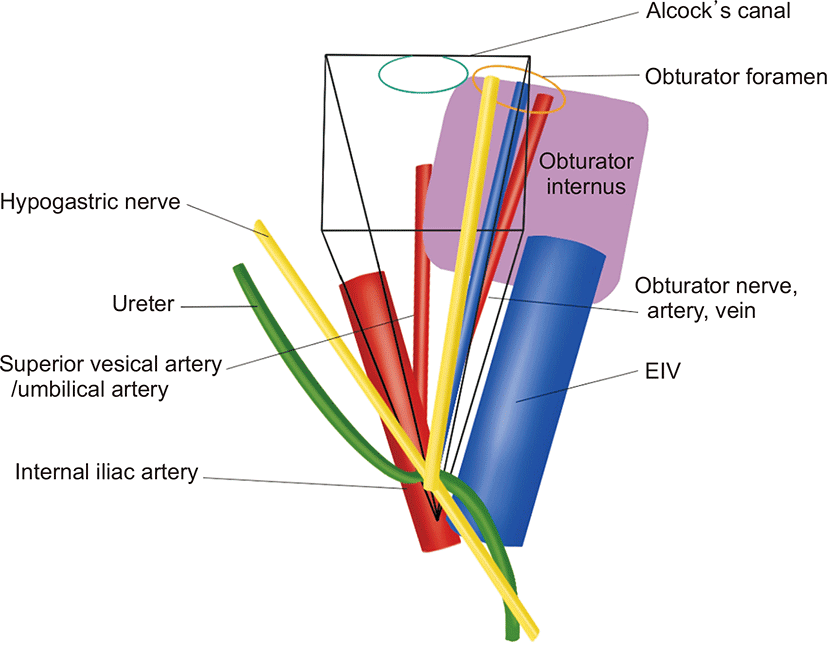
LPLN metastasis in rectal cancer is associated with lateral lymphatic spread to the rectum. The LPLNs associated with lateral lymphatic flow are the common and internal iliac, obturator, and middle and inferior rectal lymph nodes and are located in the space boundaries created by the internal and external iliac vessels, obturator internus muscle, and parietal pelvic fascia (Fig. 1). These lymph nodes are the more common metastatic sites of low rectal cancer. The incidence of LPLN involvement in low rectal cancer varies from 10% to 25% [16], with 7% of patients harboring occult micro-metastases in the lymph nodes, which are not detected on conventional histopathology [17]. Moreover, the presence of metastases in the LPLNs in the absence of positive nodes along the inferior mesenteric artery has been reported in up to 15% of patients. The closer the low rectal cancer to the anus, the higher the risk of lateral node involvement (above peritoneal reflection, 8.2%; below peritoneal reflection, 14.9%); moreover, the higher the T-staging, the greater the risk of metastases to the LPLNs (T2, 7.1%; T3, 17.9%; and T4, 31.6%) [18].
Historically, the standard strategies in Eastern and Western countries have resulted in similar LR rates [19], which prompted Western countries to rely on nCRT to sterilize the lateral compartment. LPLN, however, has been reported as the primary LR site even after nCRT [4,6,20,21]. A recent multinational retrospective cohort study based on a pooled analysis of patients from seven countries, with 12 involved hospitals, showed that 54% of patients had LR in the lateral component [6]. Among 1,216 patients, 968 patients (79.6%) received nCRT. The size of the LN on MRI is an important predictor of LR. The size of the lateral nodes in both short and long axis was significantly associated with the 5-year lateral LR (LLR) rate. A long-axis LN diameter of >7 mm was associated with significantly greater risk, while a short-axis LN diameter of >5 mm was associated with significantly greater risk of LLR.
A retrospective Korean study including 443 patients with stage II and III rectal cancer [22] reported that 11.9% of patients developed LR. Among the patients with LRs, 37.7% developed cancer recurrence in the lateral pelvic area. However, an LPLN size of 10 mm was not a significant risk factor of lateral pelvic LN recurrence in this study. The other Korean studies reported a lateral pelvic recurrence of up to 82.7% [4,20] and showed that LPLN recurrence is common in patients treated with nCRT and TME; meanwhile, the LPLND area is extremely limited in some selected patients. Both studies showed that an LPLN size of >10 mm was significantly associated with high LLR rates. A European study also reported that 64.3% of patients with advanced low rectal cancer (≤8 cm from the anal verge) who underwent nCRT and TME had a recurrence in the lateral compartment [23]. They reported that the long-axis LPLN diameter did not influence the LLR rates; however, patients with a short-axis LN diameter of >10 mm had a significantly higher 4-year LLR rate (33.3%) than those with short-axis diameter of <10 mm (10.1%, P=0.03).
In previous studies, more than half of the patients who experienced locoregional recurrences had a recurrence in the lateral compartment; even in patients with recurrent diseases, half did not develop distant metastases after undergoing nCRT and TME. Therefore, nCRT and TME are not effective treatments in patients with enlarged LPLN. Moreover, the subgroup of patients who require more than nCRT and TME should be considered to improve the locoregional recurrence rate.
Criteria for LPLND in Patients Who Received nCRT: Size before nCRT vs. Change after nCRT?
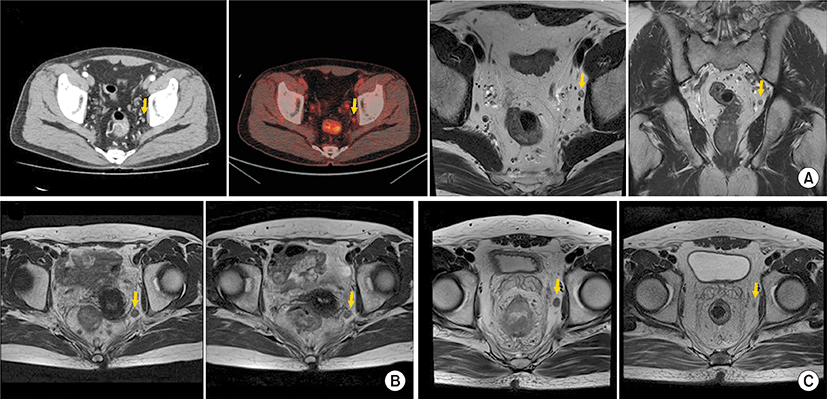
The LPLN size before treatment is the main factor for predicting lateral pelvic recurrences and LPLN metastasis and used as one of the criteria for determining the need for LPLND [24]. For the diagnosis of LPLN metastasis, MRI, CT, and positron emission tomography are used (Fig. 2). The imaging modalities used to diagnose LN including LPLN metastasis and the metastatic LN size varied among studies (Table 1) [6,13,15,20,23,252627-28]. In addition, whether the LPLN size pre- or post-nCRT would be used as more optimal criteria remains controversial.
A study by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) compared the short-axis cutoff values of 5 and 10 mm determined on a preoperative MRI were compared in terms of accuracy, sensitivity, and specificity [29]. The above study found that the 5 mm criterion was superior and had higher sensitivity (72.6% vs. 19.5%), lower specificity (54.7% vs. 96.4%), and higher accuracy (63.7% vs. 57.7%). A study by JSCCR insisted that a short-axis LPLN diameter of ≥5 mm measured through an MRI was more predictive of LPLN metastases than the histopathological grade, lymphatic invasion, perirectal LN metastases, and distant metastases [30]. A study in patients who received nCRT found that a short-axis LPLN size of 7 mm was important in predicting the 5-year recurrence-free survival. In patients with a short-axis LPLN size of >7 mm, those who underwent LPLND showed improvement in the survival compared with those who did not undergo this procedure (85.7 % vs. 56.8%, P=0.0038) [11].
The administration of nCRT aimed to decrease the tumor extent in the pelvic lymph node as well as the primary tumor. Patients who achieved a good response to nCRT showed low incidence of lymph node metastasis and better oncologic outcomes than those with poor response [3,5]. Therefore, the change in the LPLN size has been considered as an indication for LPLND.
The Lateral Node Study Consortium reported that the LPLND significantly the reduced 5-year lateral pelvic recurrence and distant recurrence (DR) rates in patients with a short-axis LPLN diameter of >7 mm on pre-nCRT MRI [6]. In the subgroup analysis, they evaluated the effect of restaging cancer with MRI in 741 patients who received nCRT and underwent restaging MRI [31]. Among 741 patients, 651 underwent nCRT with TME and 90 underwent nCRT with TME and LPLND. Compared with nCRT with TME alone, nCRT with TME and LPLND in these unresponsive internal nodes resulted in a significantly lower LLR rate of 8.7% (hazard ratio, 6.2; 95% confidence interval, 1.4–28.5; P=0.007) in patients with LPLN diameters of ≥7 mm on primary MRI and >4 mm on restaging MRI. They insisted, however, that lateral lymph node dissection (LLND) can be avoided in patients whose LPLN size decreased, from a short-axis diameter of ≥7 mm on primary MRI to diameter of ≤4 mm on restaging MRI, as there was absence of LLRs.
However, with the same size criterion, Kim et al. [28] reported that patients whose short-axis LPLN diameter of ≥7 mm on pre-nCRT MRI decreased to <4 mm after nCRT was associated with a lower incidence of LR, but the degree of DR risk remained the same in 798 rectal cancer patients treated with nCRT. In the entire cohort, LPLN sampling did not show improvement in local control.
Akiyoshi et al. [32] evaluated whether post-nCRT change in LPLN size would be an indication for LPLND in 77 patients who had locally advanced low rectal cancer with a long-axis LPLN diameter of >7 mm and received nCRT. After the nCRT, restaging MRI and LPLND were performed. Before and after nCRT, patients with short-axis LPLN diameters of >8 mm and >5 mm, respectively, showed higher LPLN metastasis rate. LPLN metastasis was associated with poor 3-year relapse-free survival (RFS), but the response of LPLN to nCRT was not associated with relapse-free survival. Patients with a >60% reduction in the volume of LPLN after nCRT did not show LPLN metastases. Authors, therefore, concluded that the responsiveness of LPLN after nCRT is not a suitable method for measuring LPLN metastasis.
A study retrospectively analyzed 580 patients with advanced low rectal cancer who underwent nCRT followed by TME with LPLND [15]. They divided patients into three groups based on the LPLN size (5 mm) before and after nCRT: no suspected LPLN group (LPLN <5 mm pre- and post-nCRT), responsive LPLN group (LPLN ≥5 mm pre-nCRT but <5 mm post-nCRT), and persistent LPLN group (LPLN ≥5 mm pre- and post-nCRT). The persistent group had significantly poorer LPRFS, LRFS, RFS, and overall survival (OS) than the responsive and no suspected LPLN groups (P<0.05). The responsive group tended to have poorer LPRFS, LRFS, RFS, and OS than the no suspected LPLN group, and the differences in RFS and OS between the two groups were not significant (P>0.05). Based on these results, the subgroup whose LPLN was responsive to nCRT may not benefit from LPLND. They concluded that patients who had persistent LPLN (LPLN ≥5 mm pre- and post-nCRT) would be an indication for LPLND.
Similar conclusion, in which a responsive LPLN would not be a definite indication for LPLND, was obtained by a retrospective, multicenter (three Korean hospitals), cohort study that analyzed 66 patients who had locally advanced low rectal cancer (below the peritoneal reflection), with radiologically suspected LPLN (>5 mm) [33].
Recent data of the largest Western study from MD Anderson Cancer Center proposed that patients with rectal cancer and clinical evidence of LPLN metastasis and a post-nCRT LPLN diameter of ≥5 mm need to be considered for LPLND [34]. Among 64 patients who were included, 33 (51.6%) had LPLN metastasis after nCRT, and this occurred in all patients with a post-nCRT LPLN diameter of ≥5 mm.
Most of the previous studies that compared the changes in pre-/post-nCRT LPLN size and recurrences presented that LPLND should be considered in patients with persistent clinically metastatic LPLN. However, patients with responsive LPLN rarely benefited from LPLND.
Impact of LPLN Metastasis on Oncologic Outcomes
Whether LPLN metastasis is regarded as regional metastasis or distant metastasis remains controversial. The current American Joint Committee on Cancer Cancer Staging Manual categorized internal iliac lymph node metastasis from rectal cancer as regional metastasis, while metastases to other lateral nodes such as obturator, external iliac, and common iliac are defined as distant metastasis [35]. LPLN metastasis is regarded as a regional disease, and LPLND is the recommended management in Japan; in the Western countries, it is considered a distant metastasis and usually recommended systemic treatment [6,12,28].
Therefore, the prognostic significance of LPLND on LR and survival remains undefined especially in patients treated with nCRT (Table 2) [6,9,2526-27,36,37]. LPLND was reported to reduce the LR rate and improve the OS rate [6,38]. However, some studies reported that LPLN metastasis showed poor oncologic outcomes even after LPLND because patients with LPLN metastasis had a high distant metastasis rate [28]. A multicenter retrospective study involving 12 hospitals in seven countries reported the beneficial effects of LPLND in the LR, LLR, DR, and 5-year cancer-specific survival (CSS) rates (P=0.042, 0.005, 0.028, and 0.032, respectively) compared with the absence of dissection in patients with a short-axis lateral node diameter of ≥7 mm on pre-nCRT MRI [6,31].
The location of metastatic LPLN was suggested as a determinant factor; the study by Akiyoshi et al. [39] evaluated the 5-year survival in 8,933 patients from a Japanese nationwide database of patients with low rectal cancer. In this study, selective LPND had a better prognosis than R0 resection of M1 rectal cancer. The 5-year OS of patients with internal iliac LN metastasis was similar to that of patients with N2a disease.
Patients with metastasis beyond the internal iliac LPLN had worse 5-year OS (29% vs. 45%) and CSS (34% vs. 49%) than those with internal iliac LN metastasis. A multicenter trial involving participating centers reported that patients with a post-SA node diameter of >6 mm in the obturator compartment had significantly higher 5-year DM rate (37% vs. 15% respectively; P=0.031) and lower 5-year CSS (79% vs. 96%, P=0.005) than those with a post-SA node diameter of ≤6 mm. No significant difference was observed in the DM and CSS rates among patients who underwent LLND [40].
Status of Minimally Invasive Approach for LPLND
LPLND is a technically demanding procedure; it might have a long operation time and a high risk of blood loss and postoperative complications. For LPLND, a minimally invasive approach has been used as it allows a magnified view and may facilitate access to the lateral pelvic compartment. In addition, introduction of an indocyanine green (ICG) dye during a near-infrared fluorescence imaging improves the LPLND identification [41,42].
Laparoscopic LPLND had less blood loss and shorter length of hospital stay, but had complication rates similar to those of open LPLND [43,44]. However, laparoscopic LPLND is even more difficult to perform due to poor ergonomics and the narrow lateral pelvic compartment, limiting the insertion of instruments [43,44]. Robotic LPLND has certain advantages compared with laparoscopic LPLND: improved ergonomics, improved visualization, and articulated instrument, which is useful for the precise dissection of the region [45].
Compared with open or laparoscopic LPLND, robotic LPLND had more favorable short-term and long term outcomes even for Western patients [46] The postoperative complication rates of minimally invasive LPLND including laparoscopic and robotic LPLND was reported to be variable. Ogura et al. reported that 9.3% of the patients developed major complications after undergoing laparoscopic LPLND [44]. Bae et al. [47] and Kim et al. [48] reported that 28% to 34% of patient developed surgical complications after undergoing laparoscopic or robotic LPLND. Kim et al. [48] compared laparoscopic LPLND (28%) with robotic LPLND (34%) and showed similar surgical complication rates. The incidence of functional impairment varied in terms of definition and diagnostic criteria [49,50]. Sexual and urinary dysfunction is major complications related with LPLND. Even after laparoscopic LPLND, urinary retention was reported in 5% to 78%; however, robotic LPLND showed improved functional outcomes, and experienced centers reported relatively low urinary and sexual dysfunction rates. Although the data are limited, robotic LPLND may have better postoperative functional outcomes than open or laparoscopic LPLND. Kim et al. [48] presented low incidence of urinary retention in the robotic LPLND group than in the laparoscopic LPLND group (4% vs. 20%, respectively).
Conclusion
Even after nCRT, LPLN is the main and troublesome site of rectal cancer recurrence. Although there are still controversies regarding the oncologic benefit of LPLND in patients with rectal cancer with metastatic LPLN who underwent nCRT, previous studies indicated that LPLND would be helpful for a subgroup of rectal cancer patients. The effect of LPLND on reducing LR is evident; however, its impact on distant metastasis control or association with systemic recurrence is still debatable. Existing evidence reporting the oncologic benefit of LPLND remains insufficient, and randomized controlled trials (RCTs) are warranted to confirm this finding. Before conducting RCTs, some aspects of LPLND should be clarified or studied in advance.
The modalities and criteria for diagnosing LPLN metastasis should be standardized. The indication for LPLND is an important issue that needs to be discussed. It remains debatable whether pre-nCRT measurement or response to nCRT should be considered as an indication for LPLND. In addition, technical demand is one of the limitations of LPLND, making it difficult to conduct RCTs.
A minimally invasive approach especially robotic LPLND would facilitate review technical requirement of participants. The introduction of ICG during near-infrared fluorescence imaging is helpful to perform a complete and secure LPLND.
Previous studies evaluating the oncologic impact of LPLN metastasis or role of LPLND in rectal cancer after nCRT are increasing. However, caution must be observed when treating patients with LPLN metastasis. Hence, proper selection of suitable patients must be performed in an organized trial.