Introduction
Coronary artery spasm or variant angina is rare and difficult to detect on routine preoperative evaluation. The features of ischemic episodes due to variant angina differ from those of typical angina. Such an episode occurs when a patient is resting or in the early morning and is not associated with exercise [1]. Moreover, silent ischemic episodes are frequent, and syncope is occasionally present [2]. In severe cases, transient ST-segment elevation and arrhythmias such as ventricular tachycardia and ventricular fibrillation can follow. Coronary artery spasms can occur during surgery under general or regional anesthesia. Since patients are unconscious and cannot complain of symptoms during general anesthesia, early detection of coronary artery spasms is difficult, which could lead to severe bradycardia or cardiac arrest. We report a case of a patient having variant angina with severe hypotension and ventricular fibrillation during general anesthesia.
Case
A 70-year-old female was scheduled for laparoscopic cholecystectomy for chronic cholecystitis. Her height and weight were 153.0 cm and 82.1 kg, respectively. Her body mass index was 35.1 kg/m2. The patient had hypertension and type 2 diabetes mellitus and was a hepatitis B carrier. The patient also had prurigo nodularis and was taking 5 mg prednisolone per day. The patient had experienced chest pain 15 years previously and has been taking 100 mg aspirin daily since then. The patient reported that she had not undergone any diagnostic tests, such as angiography for coronary artery disease, following the episode of chest pain. There were no specific findings on preoperative EKG or transthoracic echocardiogram.
After the patient was transferred to the operating room (OR), standard monitors, including noninvasive blood pressure, EKG, pulse oxygen saturation (SpO2), and bispectral index, were applied. The initial noninvasive blood pressure and heart rate (HR) were 137/62 mmHg and 71 beats/min, respectively. EKG showed a normal sinus rhythm, and SpO2 was 100%. Anesthesia was induced uneventfully after sequential administration of glycopyrrolate (0.2 mg), midazolam (3 mg), 1% propofol (80 mg), fentanyl (50 μg), and rocuronium (32 mg). Following intubation, arterial cannulation was performed, and continuous arterial blood pressure (ABP) monitoring was initiated. Results of arterial blood gas analysis were within the normal range: potential of hydrogen (pH), 7.401; partial pressure of carbon dioxide (pCO2), 33.6 mmHg; partial pressure of oxygen (pO2), 260.9 mmHg; base excess (BE), −4.4 mEq/L; and arterial oxygen saturation (SaO2): 97.7%. Anesthesia was maintained with 1%–2% sevoflurane and intermittent fentanyl injection.
The laparoscopic procedure was conducted uneventfully for 15 min, and carbon dioxide (CO2) insufflation was discontinued. During the surgery, EKG showed a normal sinus rhythm, ABP ranged from 96/54 to 183/91 mmHg, and HR was 80–95 beats/min. SpO2 was maintained at > 99%, and end-tidal CO2 tension was between 31 and 35 mmHg. Five minutes after the beginning of wound suturing, her ABP and HR were 75/49 mmHg and 91 beats/min, respectively, and 5 mg ephedrine was injected intravenously. However, systolic ABP decreased to approximately 50 mmHg. After an additional dose of 5 mg ephedrine, tachycardia with an HR of 130 beats/min was noted. Despite starting norepinephrine infusion, ABP did not increase, and ST-segment elevation (2.5 mm) followed by ventricular fibrillation was noted in the EKG. Chest compression was initiated immediately, and spontaneous circulation (ROSC) was returned after one cycle (2 minutes) of cardiopulmonary resuscitation without defibrillation. Blood samples were collected for cardiac enzyme tests, and 12-lead EKG monitoring was taken.
Despite ROSC, systolic ABP was still low (approximately 60 mmHg). Therefore, an epinephrine infusion was started. ST-segment elevation in the territory of the left anterior descending coronary artery was observed on 12-lead EKG (Fig. 1). Results of post-ROSC arterial blood gas analysis were as follows: pH, 7.283; pCO₂, 41.4 mmHg; pO₂, 342.5 mmHg; base excess, −7.5 mEq/L; and SaO₂, 97.9%. Cardiac enzyme levels at the time of the event were within the normal ranges (high-sensitivity cardiac troponin T, 0.004 ng/mL and a myocardial fraction of creatine kinase, 0.7 ng/mL). At 10 minutes after the event, vital signs began to stabilize, and ST-segment elevation was normalized. The patient was transferred to the intensive care unit under intubation with continuous infusion of epinephrine and norepinephrine (ABP, 93/51 mmHg).
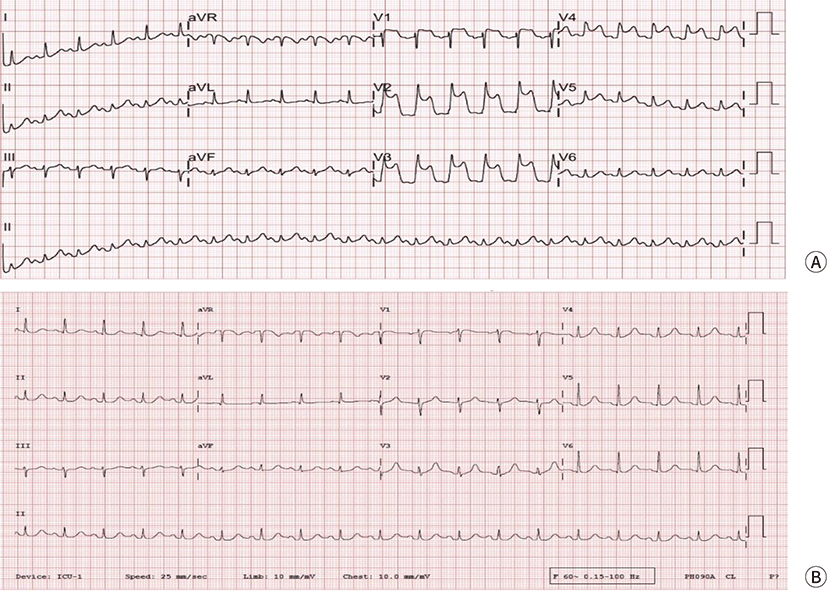
The operation time was 45 minutes, and the anesthesia time (from arrival in the OR to transfer to the intensive care unit) was 100 minutes. Total intake was 1000 mL of crystalloid fluid, and total output was 100 mL (20 mL of blood loss and 80 mL of urine output) in the OR.
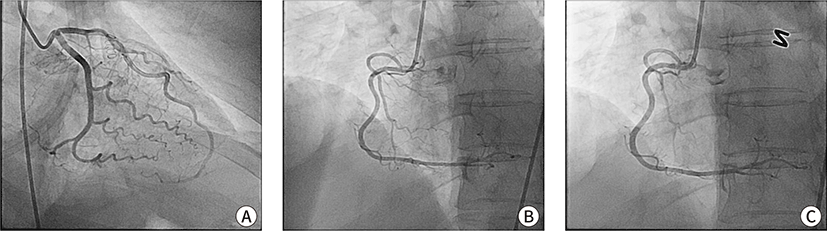
Emergency coronary angiography (CAG) was performed 1 hour later, which revealed no stenotic lesions (Fig. 2). To avoid another potentially refractory coronary spasm, a vasodilation test using nitroglycerin (NTG) was performed during CAG. The right coronary artery showed reactive vasodilation following intracoronary administration of NTG (Fig. 2). Therefore, the patient was diagnosed with variant angina. Vital signs stabilized after CAG, and epinephrine and norepinephrine were tapered and stopped the next morning. The patient was extubated and transferred to the general ward the next day and discharged 6 days later with a prescription for a calcium channel blocker and NTG to be used when necessary.
Discussion
In the present case, we experienced coronary vasospasm in a patient with undiagnosed variant angina. The first report of the variant of angina pectoris was by Prinzmetal in 1959 [3]. The pain of variant angina is not provoked by exercise or excessive workload on the heart but occurs spontaneously at rest or during ordinary activity. Coronary vasospasm is accompanied by a characteristic change in the EKG: significant ST-segment elevation and reciprocal ST-segment depression [4]. Moreover, transmural ischemia caused by coronary artery spasms may be silent but serious, leading to lethal arrhythmias such as ventricular tachycardia and fibrillation [5]. It occurs in relatively young individuals with low coronary risk and can be confirmed by provoking local vasospasm by vasoconstrictors such as ergonovine and acetylcholine during CAG [1,6,7].
During general anesthesia, patients cannot complain of the characteristic symptoms. Therefore, ST-segment elevation on EKG is the only clue for recognizing coronary vasospasm. However, sometimes life-threatening arrhythmia can occur without ST-segment change or happens too quickly before anesthesiologists can recognize the changes in the EKG [8,9]. Therefore, careful vigilance and early detection of events are critical for patient management.
Among the drugs used for anesthesia, coronary artery vasospasm has been reported to be caused by vasoconstrictors such as ephedrine, phenylephrine, norepinephrine, and epinephrine [10,11]. Hence, careful observation of any changes in the EKG after the administration of these drugs is necessary. In the present case, the initial decrease in ABP might not have been caused by the spasm. However, we used ephedrine to increase the ABP, which could have caused the spasm. Another potential cause of spasms is abdominal manipulation. At the end of CO2 insufflation, the surgeons pressed down the abdominal wall to pull the gas out, which could have provoked a vagal-mediated reflex [12,13]. Coronary artery spasms may be affected by the autonomic nervous system, and increased sensitivity of the vascular smooth muscles can cause an attack [14].
Initially, we considered the possibility of coronary artery occlusive disease due to a definite ST-segment elevation and a history of chest pain and aspirin consumption. In the present case, the patient had not undergone any diagnostic tests for coronary disease despite a history of chest pain. Similarly, undiagnosed variant angina can be overlooked when coronary vasospasm occurs during surgery. Despite the presence of many risk factors for cardiovascular disease, the preoperative transthoracic echocardiogram was normal, and the possibility of underlying heart disease was believed to be low. Therefore, variant angina remained undiagnosed, and ephedrine was used without the awareness of the potential risk of coronary artery spasm. Since the event was diagnosed as coronary vasospasm, NTG or calcium channel blockers (CCB) would have been helpful in relieving the artery. Generally, the primary treatment for variant angina is NTG and CCB since both of these medications have vasodilatory effects [1]. NTG is a vasodilator that acts independently of vascular endothelial cells, and CCB acts on the vascular smooth muscles. Particularly, the response to these drugs is stronger in variant angina, which is not associated with other factors that reduce intravascular volume, such as vascular stenosis. Therefore, we believe that the rapid administration of these drugs is important.
In addition, sufficient post-surgical evaluation should be conducted for an exact diagnosis so that the patient can continue treatment if necessary. Patients should be educated about the importance of explaining their history to future anesthesiologists. The suspicion of coronary artery spasms can affect the early detection of an event and the choice of drug. Therefore, detailed preoperative history and evaluation are essential.
In conclusion, coronary artery spasms caused by variant angina can result in fatal arrhythmias and myocardial ischemia. Therefore, caution should be exercised during anesthesia. Furthermore, variant angina cannot be detected easily during anesthesia since the typical symptoms and signs are masked. Therefore, careful vigilance by the anesthesiologists, prompt response, and treatment are essential.
