Introduction
Prader-Willi syndrome (PWS) is a rare multisystem genetic disorder that is recognized as the most common genetic cause of obesity [1]. Its incidence ranges from 1 in 10,000 to 1 in 30,000 births [2]. PWS results from the absence of imprinted genes in the paternally derived PWS/Angelman syndrome region of chromosome 15q11.2–13.
Clinical manifestations of PWS include poor sucking and swallowing difficulties accompanied by infantile hypotonia, followed by delayed motor milestones. Patients with the condition begin to experience hyperphagia and obesity in early childhood, along with reduced physical activity. They also exhibit abnormal body composition characterized by increased fat mass and reduced lean body mass, as well as a low metabolic rate potentially leading to severe obesity [1,3,4]. Developmental delay/intellectual disability, learning difficulties, behavioral problems, and autistic features are common [5,6]. Characteristic facial features of PWS include narrowing of the forehead, almond-shaped eyes, small chin, and high-arched palate [3]. Endocrine abnormalities, such as growth hormone deficiency, hypopituitarism, hypothyroidism, and hypogonadism, may also be present [3]. The PWS phenotype is currently believed to result from the complex dysregulation of hypothalamic control [7].
The management of PWS necessitates a multidisciplinary team approach that includes a neonatologist, medical geneticist, pediatric endocrinologist, dietitian, orthopedist, and rehabilitation therapist [6,8]. Early diagnosis and intervention are crucial for preventing morbid obesity, which is key to managing patients with PWS. Due to morbid obesity, individuals with PWS may experience numerous complications, such as type 2 diabetes mellitus (T2DM), metabolic syndrome, obstructive sleep apnea, respiratory failure, thromboembolism, pulmonary hypertension, and right heart failure. These complications contribute to a high mortality rate relative to the general population [7,9–12]. In this context, the present review covers the genetic and endocrine mechanisms of obesity and the current therapeutic strategies for managing obesity in PWS.
Hyperphagia and Obesity Phenotype in Prader-Willi Syndrome
Patients with PWS experience poor sucking and feeding difficulties during infancy, followed by uncontrolled hyperphagia and a lack of satiety. This can lead to rapid weight gain and obesity beginning at 2 to 3 years of age. Progressive food-seeking behavior and hyperphagia are observed in association with constant and inexorable hunger, which can lead to life-threatening obesity in adults with PWS [10]. Individuals with PWS often exhibit behavioral problems related to aggressive and obsessive food-seeking, including hoarding food, foraging, and stealing food or money to purchase food [7]. These abnormal behaviors can cause lifelong distress for patients and their families and may negatively impact social adaptation, occupational performance, and quality of life.
The obesity phenotype in PWS is distinguishable from other common forms of obesity [6]. Patients with PWS typically have lower muscle mass than individuals with simple obesity, which results in lower resting energy expenditure [13]. In contrast, patients with PWS have higher fat mass than body mass index (BMI)-matched individuals with common obesity [13,14]. Typically, individuals with PWS exhibit an excessive accumulation of subcutaneous fat in the trunk and proximal extremities in conjunction with relatively low visceral adiposity, which is responsible for higher insulin sensitivity compared to BMI-matched populations with common obesity [14].
Genetics of Prader-Willi Syndrome
Three distinct genetic mechanisms are responsible for PWS: approximately 65% to 70% of cases result from paternal deletion of 15q11.2–13, 20% to 30% are caused by maternal uniparental disomy of chromosome 15, and the remaining 2% to 5% of cases result from imprinting center defects or chromosome 15 rearrangement [15–19]. The paternally expressed PWS region contains several genes, including MKRN3, MAGEL2, NECDIN, and small nucleolar RNA genes [6,20]. SNORD116, a small nucleolar RNA gene, is known to be the critical gene for most PWS phenotypes [21,22]. Depletion of SNORD116 has been demonstrated to cause an imbalance in the neuromodulatory systems of the hypothalamus, leading to abnormal food intake behavior and sleep problems in a mouse model that mimics the clinical manifestations of PWS [21].
Hypothalamic Abnormalities in Prader-Willi Syndrome
Structural brain alterations, including a scarcity of oxytocin neurons in the hypothalamus and reduced fractional anisotropy in neuron fibers, have been linked to uncontrollable hyperphagia and a lack of satiety [7,23,24]. Mouse models with disrupted SNORD116 expression in the mediobasal hypothalamus have mimicked the hyperphagic behavior observed in PWS [25]. Furthermore, imaging studies have shown an increased hypothalamic response to food stimuli and diminished coupling between the ventral striatum and limbic structures [26,27]. These findings suggest that structural or functional dysregulation of the hypothalamus plays a critical role in the hyperphagia and obesity associated with PWS.
Endocrine Alterations in Prader-Willi Syndrome
The mechanism underlying abnormal hyperphagia in PWS is not fully understood. However, several studies have demonstrated alterations in anorexigenic and orexigenic hormones in patients with PWS relative to obese individuals (Fig. 1).
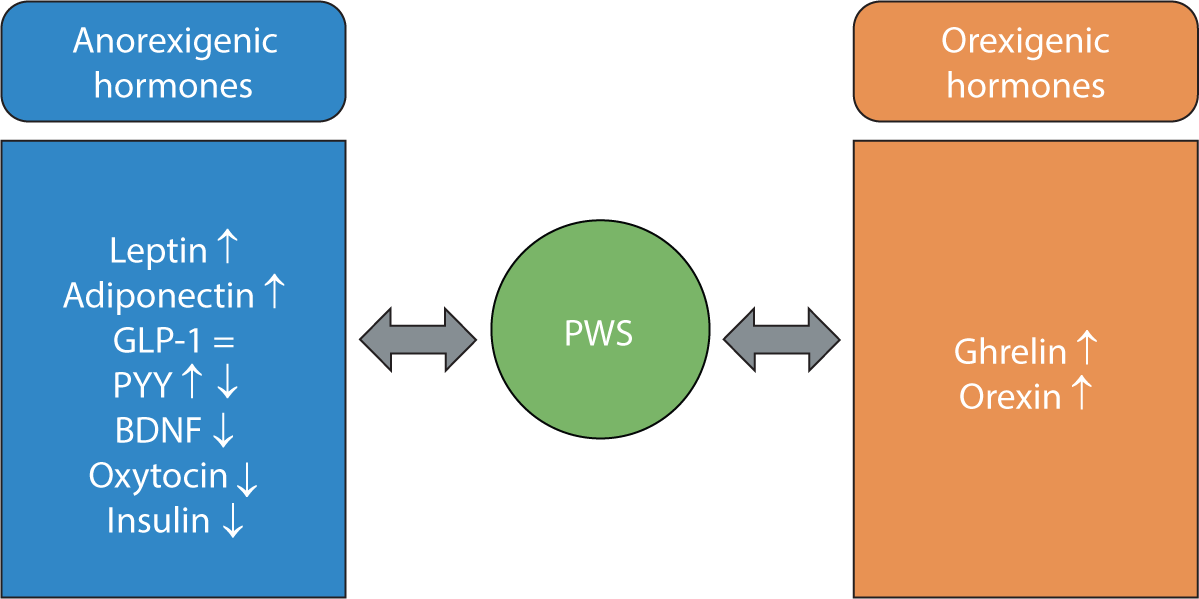
Elevated serum leptin, an anorexigenic hormone, has been reported [28]. However, leptin levels have not been shown to differ significantly between individuals with PWS and those with simple obesity [28,29]. Patients with PWS have lower circulating levels of brain-derived neurotrophic factor than individuals with simple obesity [30]. Since brain-derived neurotrophic factor acts as a satiety signal and regulates energy homeostasis, its reduced presence may contribute to the persistent hunger observed in patients with PWS [30]. Oxytocin, another anorexigenic hormone, inhibits food intake. Low levels of this hormone have also been observed in patients with PWS, suggesting a potential causal relationship with PWS-associated hyperphagia [31]. Glucagon-like peptide 1 (GLP-1) and peptide YY are secreted in response to food intake and exert anorexigenic effects. Research has found no significant difference in GLP-1 levels between individuals with PWS and those with simple obesity [32]; however, data on peptide YY levels in PWS are conflicting [7,32]. Adiponectin, released from adipose tissue, is involved in appetite modulation, energy homeostasis, and lipid and glucose metabolism, as well as insulin sensitivity and inflammation. This hormone stimulates food intake in the fasting state, and higher levels have been reported in patients with PWS compared to obese controls [33]. A well-known endocrine characteristic of PWS is relative hypoinsulinemia and low insulin resistance, despite severe obesity. This fasting and/or postprandial hypoinsulinemia may also play a role in the hyperphagia seen in PWS [7].
Among the orexigenic hormones, an elevated level of ghrelin has been observed in children and adults with PWS [34]. Two forms of ghrelin are found in circulation: acylated ghrelin (AG) and unacylated ghrelin (UAG) [19]. AG is known to stimulate hunger, and studies have shown that a high AG/UAG ratio is associated with hyperphagia and obesity in individuals with PWS [35,36]. Orexin, another orexigenic hormone, interacts with other neuropeptides to stimulate food intake. One study suggests that dysregulation of orexin may contribute to the abnormal eating behaviors observed in PWS [37].
Pharmacological Treatments for Obesity in Prader-Willi Syndrome
Pharmacological therapeutic options for patients with PWS are extremely limited. Unfortunately, no medications have yet demonstrated long-term efficacy in managing hyperphagia associated with PWS [7].
Orlistat is a pancreatic lipase inhibitor that reduces the absorption of ingested fats. This medication acts peripherally, and the resulting presence of undigested fats can alter stool consistency, potentially reducing long-term compliance [7]. A trial of orlistat in patients with PWS demonstrated modest efficacy, but poor compliance and gastrointestinal side effects were noted [38].
Metformin is commonly prescribed for patients with PWS who also exhibit insulin resistance or T2DM. A pilot study has indicated that metformin may enhance feelings of satiety and decrease food-related anxiety in certain individuals with PWS, potentially through the mechanism of improved insulin sensitivity [39].
Serotonin plays a role in reducing food intake, and serotonin receptor agonists are therefore utilized in the management of obesity. Sibutramine, a non-selective serotonin and norepinephrine reuptake inhibitor, has been considered for patients with PWS due to its promising results in those with hypothalamic obesity [40]. However, sibutramine was withdrawn from the market because of its association with adverse cardiovascular events. Lorcaserin, a selective serotonin 2C receptor agonist with high affinity, has been demonstrated effective in promoting weight loss in individuals with obesity. Moreover, it has been shown to reduce blood pressure, along with levels of total and low-density lipoprotein cholesterol, fasting glucose, insulin, and inflammatory markers [41,42]. Unfortunately, lorcaserin was also withdrawn from the market due to an increased risk of pancreatic, lung, and colorectal cancers.
Almost all children with PWS and some adults with the condition exhibit growth hormone deficiency. Growth hormone therapy is beneficial in reducing body fat, increasing lean body mass, and increasing height, which leads to improved body composition [43,44]. Several studies have demonstrated the efficacy of growth hormone therapy in addressing developmental and behavioral problems, particularly when initiated at a young age [44–46]. However, growth hormone therapy has limited effects on reducing appetite and food-seeking behavior [7].
Octreotide is a long-acting somatostatin analogue that can significantly reduce fasting ghrelin concentrations in both acylated and unacylated forms. Considering that individuals with PWS display increased serum ghrelin levels, octreotide therapy has been attempted. However, this therapy has not demonstrated significant effects on weight, appetite, or food-seeking behaviors in patients with PWS [47,48].
Topiramate acts as a modulator on sodium ion channels, gamma-aminobutyric acid (GABA) receptors, and AMPA/kainate receptors, which affects food-seeking behavior [7]. Additionally, topiramate reduces messenger RNA levels for neuropeptide Y, which stimulates food intake, increases appetite, and delays satiety [19]. In a double-blind, randomized, placebo-controlled clinical trial involving 62 adults with PWS, topiramate therapy was well tolerated and exerted a beneficial effect on eating behaviors in a dose-dependent manner, although no significant reduction in BMI was observed [49].
Emerging Treatments for Hyperphagia
Recent years have seen ongoing development of new pharmacological therapies for the management of hyperphagia and obesity in PWS.
Research has shown that GLP-1 agonist therapy is effective in managing obesity, satiety issues, and elevated blood glucose levels in patients with PWS [8,50–53]. A 6-month course of GLP-1 agonist therapy led to reduced appetite scores and lower levels of glycosylated hemoglobin (HbA1c) in a group of 10 patients with the condition, although no significant changes were observed in body weight, BMI, adiposity, or ghrelin levels [51]. In a separate study, 24 months of subcutaneous GLP-1 agonist therapy yielded reductions in BMI, waist circumference, and serum HbA1c levels in adult patients with PWS and T2DM, without serious adverse events [53].
In patients with PWS, UAG levels tend to be low, while the ratio of AG to UAG is high. Administering a pharmacologically stable amino acid form of a UAG analogue can normalize this ratio and may treat the hyperphagia associated with the condition [19,35]. In a mouse model, while no significant weight changes were observed, this treatment approach led to a reduction in waist circumference and fat mass, with no serious side effects reported [54]. A phase 2 clinical trial involving 47 patients with PWS revealed that UAG analogue therapy significantly improved food-related behaviors and showed promising metabolic outcomes [55].
Another approach involves inhibiting ghrelin O-acyltransferase, the enzyme responsible for the octanoylation of ghrelin. Inhibiting ghrelin O-acyltransferase reduces the production of AG and could lead to a decreased AG/UAG ratio, thereby helping to control hyperphagia [56,57].
An melanocortin 4 receptor (MC4R) agonist, a synthetic peptide, binds to human MC4R, leading to decreased food intake and substantial weight loss [4]. In 2020, the US Food and Drug Administration approved setmelanotide for the treatment of obesity in adults and children aged 6 years and older with monogenic obesity due to Pro-opiomelanocortin, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency [58]. Additionally, setmelanotide is under evaluation for its effectiveness in patients with syndromic obesity, such as Bardet–Biedl syndrome and Alström syndrome, as well as those with chromosomal rearrangements at the 16p11.2 locus [59–61]. However, in a phase II clinical trial (ClinicalTrials.gov: NCT02311673), setmelanotide therapy did not significantly reduce hyperphagia or body weight in obese patients with PWS [7].
Diazoxide is an adenosine triphosphate-sensitive potassium (KATP) channel agonist used in the treatment of hyperinsulinemic hypoglycemia. It has been shown to help manage obesity in PWS by downregulating insulin secretion, reducing the synthesis and secretion of hypothalamic appetite-stimulating neuropeptides such as neuropeptide Y and Agouti-related protein, increasing GABAergic neuronal excitability, and activating KATP channels in adipocytes [19]. One study reported that 14 weeks of oral diazoxide administration was associated with significant reductions in hyperphagia and aggressive behaviors [62]. Additionally, diazoxide treatment in adolescent and adult patients with PWS was associated with decreased fat mass, waist circumference, and improvements in lipid profiles and insulin resistance, although these changes did not reach statistical significance [62]. While the mechanism by which diazoxide affects hyperphagia in PWS is not fully understood, current observations suggest that diazoxide may be a potential therapeutic option, warranting further research.
Oxytocin, a hormone produced in the hypothalamic paraventricular nucleus and supraoptic nucleus, plays a role in regulating food intake and satiety [63]. Patients with PWS exhibit abnormalities in the oxytocin system [7]. Intranasal administration of carbetocin, an oxytocin analogue, has been tested in individuals with PWS and has shown a beneficial effect in reducing hyperphagia [64].
Beloranib inhibits methionine aminopeptidase 2 (MetAP2), leading to hormonal changes that decrease fat biosynthesis, enhance fat oxidation, and increase lipolysis. This compound also influences satiety in the hypothalamus. In a phase 3 clinical trial involving patients with PWS, beloranib significantly reduced food intake and promoted weight loss [65]. However, the development of beloranib was halted following the deaths of two patients from pulmonary embolism during the trial. In a preclinical study, another MetAP2 inhibitor was assessed for safety and efficacy in the treatment of diabetes mellitus and obesity; the results indicated improved safety with regard to endothelial cell proliferation and coagulation [66].
Conclusion
Once established, managing obesity in individuals with PWS is challenging due to the complex interplay of contributors to hyperphagia and obesity, including metabolic, hormonal, behavioral, and neurological factors. Consequently, early diagnosis and a comprehensive, multidisciplinary approach that includes parental education are crucial for preventing the early onset of obesity and maintaining a child’s weight within a healthy range. This involves implementing rigorous structures to limit food intake and encourage physical activity. However, sustaining lifestyle interventions over the long term often proves difficult for patients with PWS. Although no medications have consistently demonstrated effectiveness in managing obesity in PWS to date, ongoing research efforts are essential for the development of potential pharmacological therapies.
