Introduction
Heart failure (HF) is the leading cause of morbidity and mortality worldwide [1]. In Korea, the estimated prevalence of HF rose from 0.77% in 2002 to 2.24% in 2018 [2]. Sex-based stratification reveals that 600,244 women (2.3%) and 599,532 men (2.1%) are affected by this condition [2], indicating a higher prevalence in the Korean female population relative to their male counterparts. The prevalence of HF is anticipated to continue rising, as it generally increases with age. Rapid and accurate diagnosis of HF is crucial for initiating appropriate management and improving outcomes [3]. Based on left ventricular (LV) ejection fraction (EF), heart failure can be classified into three categories: HF with reduced ejection fraction (HFrEF; EF ≤40%), HF with mildly reduced EF (HFmrEF; EF 41%–49%), and HF with preserved EF (HFpEF; EF ≥50%) [4,5]. The recommended pharmacotherapies differ across these categories. Several sets of guidelines have been published for the management of HF [3,6,7], all underscoring the importance of guideline-directed medical therapy (GDMT), particularly for individuals with HFrEF. The GDMT for HFrEF includes angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), sodium-glucose cotransporter 2 (SGLT2) inhibitors, mineralocorticoid receptor antagonists (MRAs), digitalis, ivabradine, and vericiguat. In contrast, SGLT2 inhibitors are the only pharmacological treatment strongly recommended for patients with HFmrEF and HFpEF, with a weaker class of recommendation for both ARNIs and MRAs [7-10].
Sex is a key biological variable in the context of HF. Men and women exhibit distinct pathophysiologies, potentially contributing to sex-specific differences in clinical presentation and diagnosis [11,12]. HFpEF is more common in women, while HFrEF is predominantly observed in men. Notable differences between the sexes in pharmacodynamics and pharmacokinetics have also been documented [11-13]. However, current guidelines do not incorporate sex-based variations into recommendations for treating patients with HF. In this article, we review the evidence for sex-related differences in HF medications and explore pharmacological treatments for HF in consideration of these sex disparities.
Ethics statement
It is a literature database-based review; therefore, neither approval by the institutional review board nor obtainment of informed consent was required.
Sex-related considerations for heart failure medications
Historically, clinical trials have predominantly included Caucasian men [14]. Despite an increase in enrollment, women continue to be underrepresented in clinical research. In 1994, the National Institutes of Health (NIH) implemented a policy requiring that all NIH-funded human scientific and behavioral studies include women, barring a clear and compelling and rationale for their exclusion [15]. The US Food and Drug Administration introduced a regulation in 1998 titled “Presentation of Safety and Effectiveness Data for Certain Subgroups of the Population in Investigational New Drug Application Reports and New Drug Applications.” This policy mandated that new drug applications provide safety and effectiveness data that encompass key demographic subgroups, including those based on sex, age, and race [14]. Furthermore, the NIH issued guidelines in 2015 that obligated researchers to account for sex as a biological variable and to submit valid analyses based on sex, race, and ethnicity to ClinicalTrials.gov [14]. These initiatives have led to recent clinical trials that suggest differences in drug efficacy across subgroups, including sexes.
Comparatively low gastrointestinal motility, intestinal enzymatic activity, and glomerular filtration rate all influence the pharmacokinetics in women, who also tend to have a lower body weight than men [13]. Hormonal differences between the sexes influence drug receptors and responses. Additionally, differences in body composition, gastric motility, cytochrome P450 enzyme activity, drug transporter function, and excretion rates all play roles in the pharmacokinetics of drugs, impacting their absorption, distribution, metabolism, and excretion [11]. Considering the sex-related differences in pharmacokinetics, such as the impacts of smaller body size, decreased intestinal enzymatic activity, and reduced clearance rates, it is reasonable to suggest that women may be at a higher risk of medication overdose compared to male patients [16]. These distinctions underscore the need for a nuanced approach to HF pharmacotherapy, which is essential for maximizing drug effectiveness and reducing the risk of adverse effects across sexes.
Digoxin
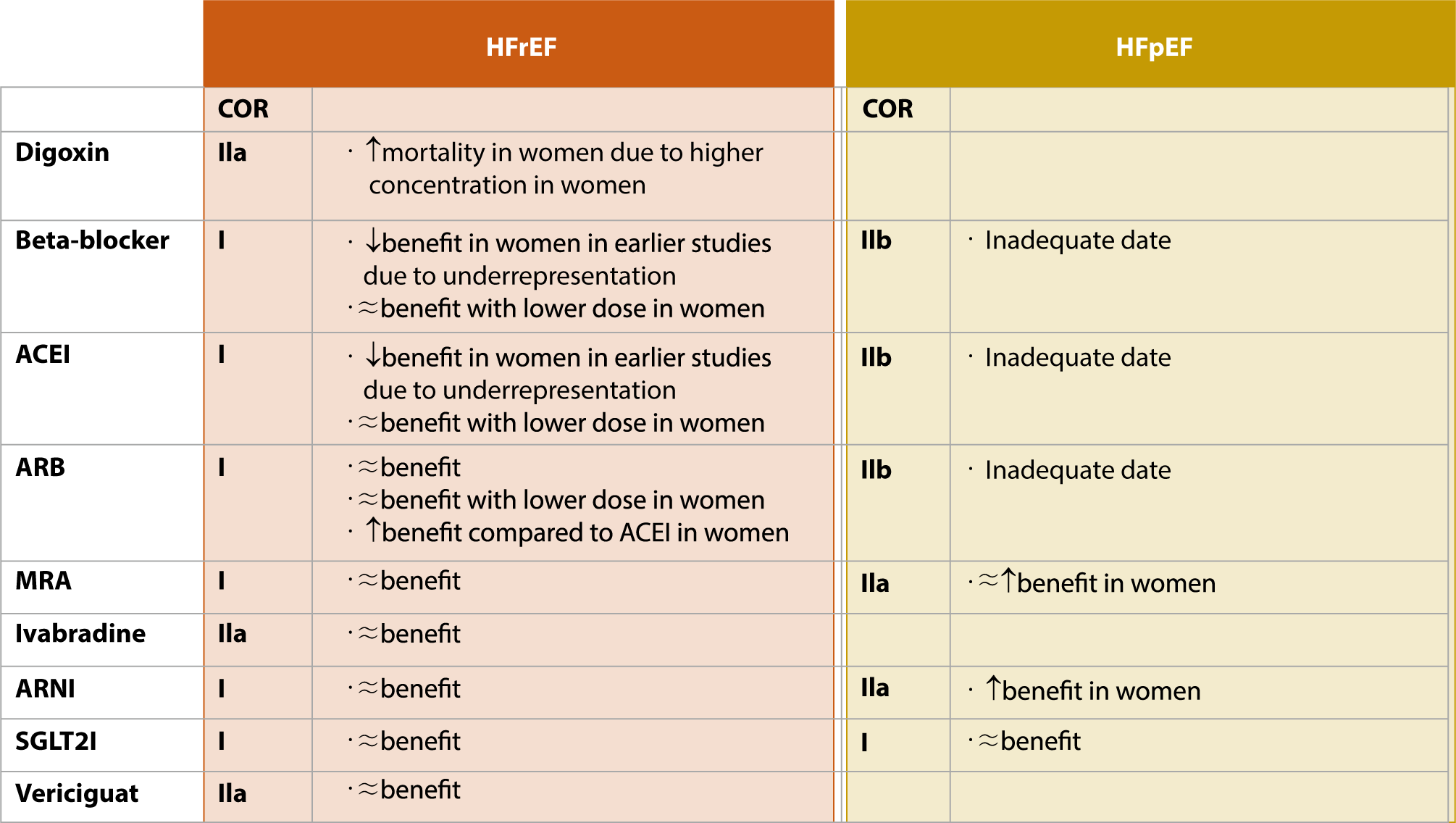
The Digitalis Investigation Group study, published in 1997, assessed the impact of digoxin on mortality and hospitalization through a randomized clinical trial that included 6,800 patients with HFrEF (EF ≤45%) [17]. The findings indicated that while treatment with digoxin did not decrease overall mortality, it did lead to a reduction in the rate of hospitalization, both overall and in cases of worsening HF [17]. Additionally, the study presented intriguing data on the sex-specific effects of digoxin, revealing that in comparison to placebo, digoxin was associated with a significantly higher mortality rate in female, but not male, patients [18]. However, the question of whether these findings are due to sex disparities remains unanswered. Further analysis of the data revealed that higher serum digoxin concentrations, which were more frequently observed in women, were associated with increased mortality [19]. The risk of death has been found to relate independently to the serum digoxin concentration, with significantly elevated risk noted in patients with concentrations of 1.2 ng/mL or higher and 1.6 ng/mL or higher [19,20]. The conclusion drawn was that the observed sex-related disparity in mortality rates associated with digoxin use was attributable to the drug concentration rather than to sex itself. In current practice, digoxin use is approached with caution and is limited to patients with HFrEF who remain symptomatic despite the optimization of GDMT [7]. The prescribed drug concentration is the same for both sexes, with target digoxin plasma concentrations maintained below 1.0–1.2 ng/mL for both men and women [21].
Beta-blockers
Most randomized clinical trials of beta-blockers in patients with HFrEF have included few female participants and minimal analysis of sex-based disparities. Initial research suggested that beta-blockers were similarly effective in women and men; however, this conclusion likely stems from the lower number of women enrolled. The US Carvedilol Heart Failure Study indicated that the impact of beta-blockers was consistent across sexes [22]. In the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure (SENIOR) trial, nebivolol was associated with a reduction in the combined endpoint of all-cause mortality or cardiovascular hospitalization in women, a benefit that was not observed in men (the P for interaction did not indicate significance) [23]. Furthermore, a meta-analysis revealed comparable reductions in mortality for men and women, with no significant sex-related differences [24,25].
Adverse drug reactions associated with the use of CYP2D6-dependent beta-blockers, including carvedilol, metoprolol, nebivolol, and propranolol, are significantly more frequent in women than in men [26]. Moreover, oral contraceptives can interact with the metabolism of metoprolol, leading to increased plasma concentrations in female relative to male patients [27]. Consequently, for several beta-blockers, women may experience the optimal therapeutic effect at doses lower than those required for men [27]. Supporting this notion, the lowest rates of death or hospitalization for HFrEF were observed at 100% of the recommended beta-blocker dose in male patients, whereas female participants experienced approximately 30% less risk at only 50% of the recommended dose. No further decrease in risk was evident at higher doses, according to a post hoc analysis of the Biology Study to Tailored Treatment in Chronic HF (BIOSTAT-CHF) study [28]. Nevertheless, no consensus exists regarding the presence of a sex-based difference in optimal beta-blocker dosage.
Beta-blockers may reduce cardiovascular mortality in patients with HFpEF [7-10]. While female sex was independently linked to the presence of diastolic dysfunction and comparatively poor clinical outcomes in a cohort of elderly individuals with HFpEF [29], sex-related disparities in the effectiveness of beta-blockers remain insufficiently examined among these patients.
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
Early trials involving ACEIs indicated a benefit exclusively for male patients, with no apparent advantage for female participants [30,31]. This discrepancy may have been influenced by the sex imbalance in these studies, in which men greatly outnumbered women—a trend also observed in studies on beta-blockers. Consequently, the limited female representation could have skewed the results unfavorably for this group. A subsequent meta-analysis of 30 studies, which encompassed substantial numbers of both male and female participants, established that ACEIs offer comparable benefits in overall mortality and the combined outcome of mortality or hospitalization for HF in both men and women with HFrEF [32]. Large-scale clinical trials later demonstrated that ARBs also provide similar decreases in adverse cardiovascular events among women and men with HFrEF [33-36]. Based on a post hoc analysis of the BIOSTAT-CHF study, Santema et al. [28] noted that the lowest risk of death or hospitalization for HF in women occurred at doses that were only half of the recommended levels, mirroring the findings regarding beta-blockers. This suggests that lower doses of ACEIs or ARBs may be warranted in women with HFrEF.
Recent data have shown that women report adverse drug reactions to ACEIs more frequently than men [37]. This sex disparity may contribute to the less frequent use of ACEIs [38]. Research using administrative databases has revealed that women treated with ARBs experienced a 31% decrease in the risk of all-cause mortality compared to those treated with ACE inhibitors [39]. In contrast, among men, the survival rates did not differ significantly between those prescribed ARBs and those given ACEIs [39]. Sex can be considered when prescribing renin-angiotensin system (RAS) blockers to patients with HF, although this requires further investigation.
Guidelines suggest that ARBs or ACEIs may reduce the risk of hospitalization or cardiovascular mortality associated with HF in patients with HFpEF [7-10]. However, insufficient research has been conducted on sex-related differences in the effectiveness of ACEIs and ARBs for treating HFpEF. Various studies have indicated that estrogen favorably modulates the RAS [40], and the cardioprotective effects of estrogen observed in premenopausal women, which are due in part to RAS inhibition, are lost following menopause [41]. Given that HFpEF is relatively prevalent among postmenopausal women, the role of RAS modulation could be particularly important for managing HFpEF in this demographic.
Mineralocorticoid receptor antagonists
For spironolactone in the Randomized Aldactone Evaluation Study (RALES) [42] and eplerenone in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) [43], no significant differences were observed between sexes in terms of prognosis for patients with HFrEF. A secondary analysis of the Aldosterone Antagonist Therapy for Adults With HF and Preserved Systolic Function (TOPCAT) trial, the TOPCAT-Americas, indicated that only women experienced a reduction in all-cause mortality when treated with spironolactone for HFpEF [44]. However, a recent meta-analysis that utilized individual patient data from the RALES, EPHESUS-HF, and TOPCAT-Americas studies showed that MRA treatment resulted in consistent reductions in the risk of adverse events for both men and women, regardless of functional class, LVEF, or other potential confounding factors [45]. Given the absence of sex-related variation in the pharmacokinetics of MRAs [41], the issue of sex-related differences in MRA treatment for HF remains unclear and warrants further investigation.
Angiotensin receptor-neprilysin inhibitor
The ARNI sacubitril/valsartan represents a frontline medical therapy for patients with HFrEF [7-10]. In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, which included patients with HFrEF, the effects of ARNI were similar for men and women [46]. The Prospective Comparison of ARNI with ARB Global Outcomes in Heart Failure with Preserved Ejection Fraction (PARAGON-HF) trial, which compared ARNI to valsartan in patients with HFpEF, found that ARNI did not significantly reduce the primary outcome when compared with valsartan [47]. However, a subgroup analysis indicated that ARNI did reduce the primary outcome in women, but not in men (P for interaction=0.016) [48]. A pooled analysis of the PARADIGM-HF and PARAGON-HF trials revealed that the therapeutic effects of ARNI vary according to LVEF, with benefits that apparently apply to patients with HFmrEF. These benefits demonstrated sex-related differences, with advantages appearing to extend to a higher LVEF in women than in men [49].
The underlying mechanism for the observed sex-related differences in the effects of ARNI on elevated LVEF remains unclear. Given that HFpEF is more prevalent in postmenopausal women, one possible explanation is modulation of the RAS, which undergoes changes after menopause. Furthermore, it is noteworthy that the LVEF is generally higher among the female than the male population [50], which implies that women may experience systolic dysfunction at higher LVEF levels compared to men [48,49]. Nevertheless, further evidence is necessary to substantiate the use of sex-specific ranges when evaluating the efficacy of LVEF-based therapies. Some researchers have noted that the representation of women in the PARAGON-HF trial was markedly higher (51.7%) than in the PARADIGM-HF trial (21.8%) [45]. Therefore, more robust evidence is needed to confirm the existence of sex-based differences in the response to ARNI treatment in HFpEF.
Sodium-glucose cotransporter 2 inhibitors
SGLT2 inhibitors, initially developed to treat type 2 diabetes, have demonstrated substantial benefits in the management of HF. These medications are effective across all HF categories, irrespective of LVEF, and exhibit clinical advantages in both men and women [51-54]. However, a recent meta-analysis of pooled data from four major randomized controlled trials on SGLT2 inhibitors suggested higher rates of primary composite outcomes in women compared to men [55]. This finding warrants further research to substantiate the hypothesis. The safety profile of SGLT2 inhibitors appears generally consistent between sexes. However, women could be more susceptible to certain adverse effects, such as urinary tract infections, due to anatomical and hormonal differences [56]. Consequently, monitoring and management of these side effects are particularly important for female patients.
Ivabradine and vericiguat
In randomized controlled clinical trials investigating ivabradine [57] and vericiguat [58], researchers observed no significant sex-related differences in the drugs’ efficacy or safety profiles among patients with HFrEF. However, the conclusions drawn from these studies are limited by the relatively small number of trials that have been conducted.
Unsolved problems and future direction
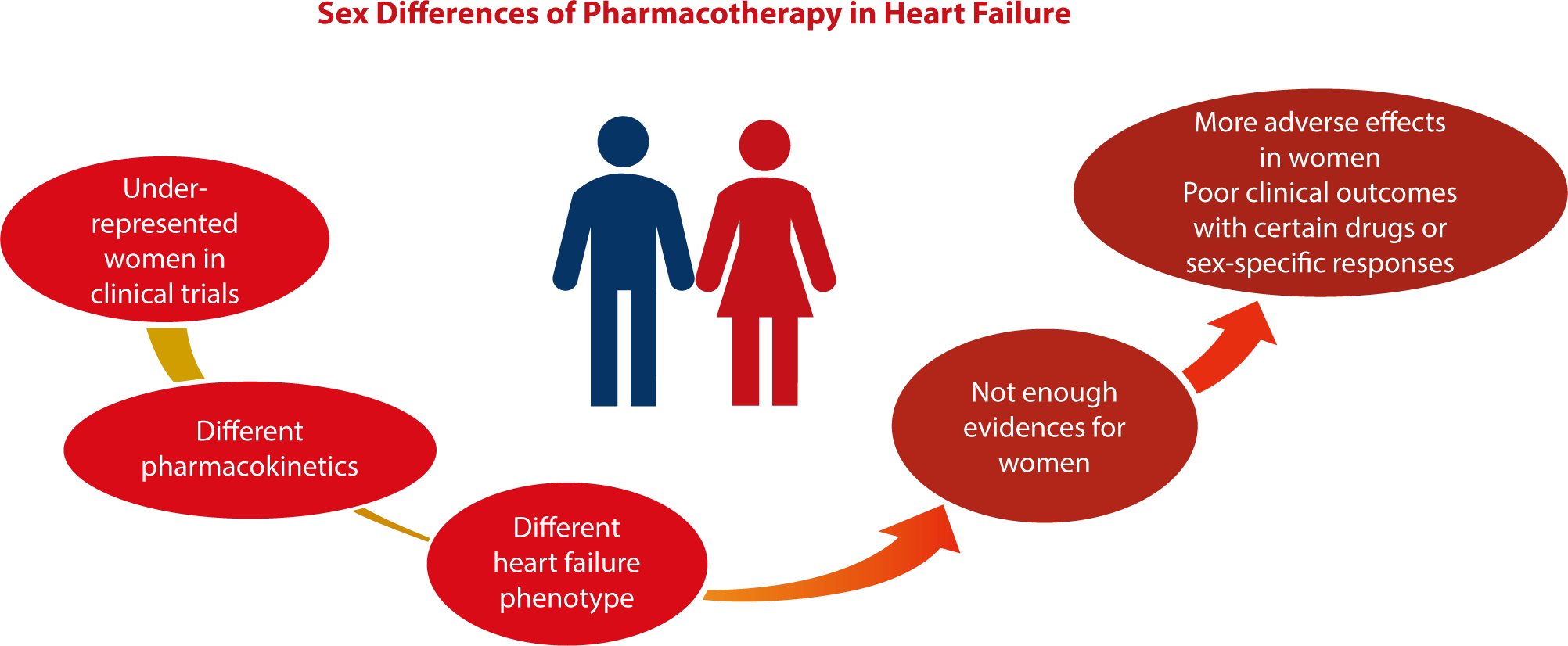
Fig. 1 provides a visual summary of sex-related differences in the efficacy of HF pharmacotherapy. Despite advances in the understanding of these differences in the context of HF medications, current guidelines frequently overlook the variations in pharmacodynamics and pharmacokinetics between men and women. Historically, women have been underrepresented in clinical trials, leading to a notable lack of data for analyzing sex disparities in drug responses. Ensuring the balanced representation of women in clinical trials is critical. Emphasis should also be placed on developing sex-specific treatment strategies for HF, regularly updating these strategies with new evidence, and implementing personalized therapeutic approaches.
Furthermore, the growing recognition of sex-related differences in HF presentation underscores the necessity for more sophisticated diagnostic tools that account for these variations. It is imperative to establish educational initiatives aimed at increasing awareness of these sex-related disparities. Moreover, fostering international collaborations can provide a broader perspective, facilitate data sharing, and pave the way for a more unified global approach to the diagnosis and management of HF that recognizes both biological and social sex and gender distinctions. Such a strategy is aligned with the core principles of precision medicine, which emphasizes the development of patient-centered approaches that are customized to the biological and genetic makeup of each individual.
Conclusion
Clinical evidence from HF trials points to sex-related disparities in pharmacotherapy for HF (Fig. 2). However, current guidelines for HF management do not provide distinct recommendations for medication based on these sex-related differences. Adopting a sex-specific pharmacological treatment strategy could be crucial in advancing precision medicine, as it would enhance our understanding of individualized patient characteristics.