Introduction
Approximately 20% of the patients with diabetes experience depression [1], and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed drugs for these patients. However, clinical and experimental trials have demonstrated that SSRIs may affect the blood glucose levels in various ways, by causing hypoglycemia [2], increasing insulin sensitivity [3], or aggravating blood glucose levels after glucose overload [4]. Fluoxetine and sertraline are especially well-studied in this field, whereas paroxetine-associated hypoglycemia has rarely been reported for the patients with type 2 diabetes. This report describes a rare case of symptomatic severe hypoglycemia that was associated with paroxetine use in a patient with type 2 diabetes complicated by diabetic neuropathy and nephropathy leading to end stage renal disease (ESRD) with hemodialysis.
Case
A 35-year-old woman was diagnosed with type 2 diabetes at the age of 18 years. She was treated with subcutaneous insulin injections (daily glargine and three times a day lispro at a total dose of 0.5 units/kg/day) without any oral hypoglycemic agents. Her recent glycated hemoglobin (HbA1c) level was 10.5%, and her body mass index was 21.0 kg/m2 (height, 1.56 m; weight, 51.1 kg). She had experienced a wound infection on her diabetic foot, proliferative diabetic retinopathy, and diabetic nephropathy leading to ESRD requiring hemodialysis three times a week since she was 30 years old. She also had severe diabetic autonomic neuropathy and diabetic gastropathy. Because of delayed gastric emptying time, she had undergone endoscopic pyloromyotomy 2 months earlier.
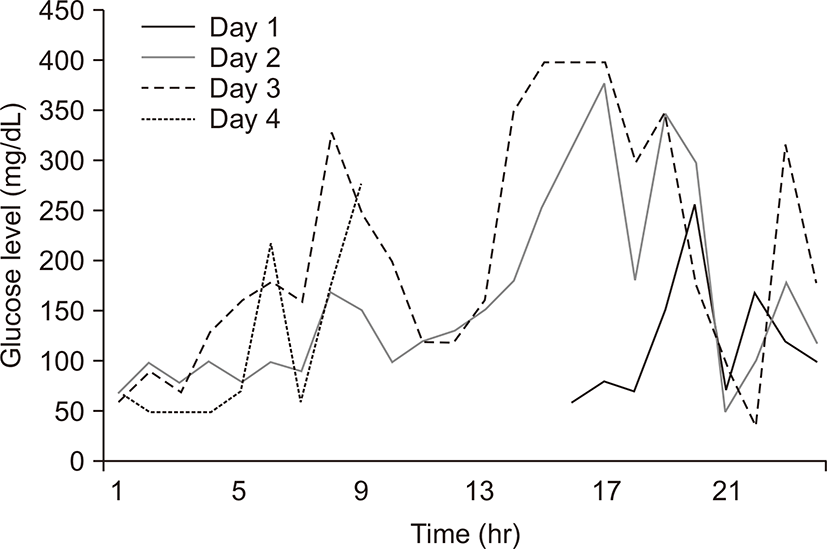
After a new onset of depression, the patient was prescribed paroxetine (25 mg per day, at night). She subsequently experienced hypoglycemic episodes approximately 4 days after paroxetine initiation. These episodes became more frequent (from one event per day or every other day, to a maximum of three events per day), with a decrease in her glucose levels to 30 mg/dL along with sweating and fatigue, which resulted in discontinuation of her insulin therapy. Although she was consuming a 1,800 kcal enough diet, under 72-hour continuous glucose monitoring (Fig. 1), these hypoglycemic episodes occurred paroxysmally without insulin therapy. At the time of a hypoglycemic event developing, her blood glucose, C-peptide, insulin, and proinsulin levels were 31 mg/dL, 1.23 ng/mL (reference range, 0.6 to 2.3 ng/mL), 4.41 μU/mL, and 1.2 pmol/L (reference range, 6.7 to 26.5 pmol/L), respectively. The HbA1c level had decreased to 6.7% (from 10.5%) over the course of 3 months. Her pituitary and adrenal functions were within the normal ranges, and she was in euthyroid state by taking levothyroxine due to hypothyroidism. There was no significant change in her body weight compared to before taking paroxetine. The test results for antibodies to insulin, insulin receptor, and glutamic acid decarboxylase were negative. Abdominal computed tomography did not reveal any pancreatic lesions, and her gastric emptying time was still delayed. After other possible causes were ruled out, the paroxetine was discontinued, after which her hypoglycemic episodes abruptly ceased. Her blood glucose levels began to rise, and she began receiving insulin again. After all, she took insulin at the same dose as before the hypoglycemic events.
Discussion
The treatment of depression in the patients with diabetes is important, because it improves their quality of life, increases treatment compliance, and facilitates better glycemic control. Although SSRIs are commonly prescribed medications in the patients with diabetes, previous studies have demonstrated that these agents may affect the blood glucose levels in various ways, by causing hypoglycemia [2], increasing insulin sensitivity [3], or aggravating the blood glucose levels after glucose overload [4].
As hypoglycemia is a severe complication associated with high morbidity and mortality, careful attention to, and early recognition of, such rare severe hypoglycemic episodes are important. Previous studies have reported that SSRIs can induce hypoglycemia by various mechanisms, including increasing insulin sensitivity [3,5], interfering with the metabolism of sulfonylureas [6], and reducing gluconeogenesis [5]. Among these mechanisms, insulin sensitivity appears to be the main effector, although the mechanism(s) underlying the association between SSRI treatments and increased insulin sensitivity remain largely hypothetical [7]. Nevertheless, fluoxetine can influence insulin sensitivity via weight loss [8], decreasing the levels of triglycerides and free fatty acids [9], altering glucose oxidation [3] or the binding affinity of insulin to the insulin receptor [3].
Few reports have described the effects of paroxetine on glucose metabolism. One case of hypoglycemia unawareness was reported in a patient with type 1 diabetes who was receiving paroxetine [10]. During 3 months of paroxetine treatment, she experienced an increased frequency of hypoglycemic episodes, and her typical warning symptoms disappeared. After discontinuation of paroxetine, her awareness of hypoglycemia was dramatically improved. The authors hypothesized that her hypoglycemic unawareness might be caused by autonomic dysfunction, which is an atypical manifestation of serotonin syndrome. Paroxetine also increased insulin sensitivity in non-diabetic patients who experienced remitted depression in a randomized trial [11], although that study did not exclude the possibility that the SSRIs lowered hypothalamus-pituitary-adrenal system activity and improved insulin sensitivity. Another study demonstrated that paroxetine had no significant or controversial effects on glycemia in healthy or diabetic mice [12]. Therefore, these results are insufficient to explain how paroxetine exerts an effect on glucose metabolism. The present case suggested that paroxetine affected the blood glucose levels, although further studies are needed to evaluate the mechanism(s) of paroxetine-induced hypoglycemia and the interactions of paroxetine with oral hypoglycemic agents or insulin.
In the present case, ESRD with hemodialysis and severe autonomic neuropathy might have made the patient more vulnerable to hypoglycemia. For example, people with renal impairment may have risk factors for hypoglycemia, such as altered drug metabolism, malnutrition, dialysis-associated problems, and impaired renal glucose release [13]. Furthermore, the presence of autonomic neuropathy attenuates the epinephrine response to hypoglycemia in the patients with diabetes after recent hypoglycemic exposure [14]. Moreover, diabetic cardiovascular autonomic neuropathy is an independent prognostic factor for the development of severe hypoglycemia in the patients with type 2 diabetes [15]. Therefore, we hypothesize that patients who have multiple co-morbidities, including renal impairment, may be more vulnerable to hypoglycemia when receiving SSRIs.
In conclusion, it is necessary to carefully monitor the blood glucose level when prescribing SSRIs to the patients with diabetes, because they may experience hypoglycemia or hypoglycemia unawareness. Furthermore, if hypoglycemia is detected, it is important to inspect a possible causative medication.