Introduction
Lymphomas of the female genital tract are extremely rare; most are secondary tumors of a disseminated disease. Lymphomas are classified into non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma, and in contrast to Hodgkin lymphoma, extra-nodal involvement is much more frequent in NHL [1]. The gastrointestinal tract and skin are the most frequent extra-nodal site, while female genital tract involvement comprises only 0.5% to 1.5% [2-4]. Primary cervical lymphoma is even rarer; therefore, diagnosis is often delayed and a consensus on its management has not yet been established. In this report, we present a case of primary cervical lymphoma diagnosed histologically as diffuse large B-cell lymphoma, and reviewed associated literatures.
Case
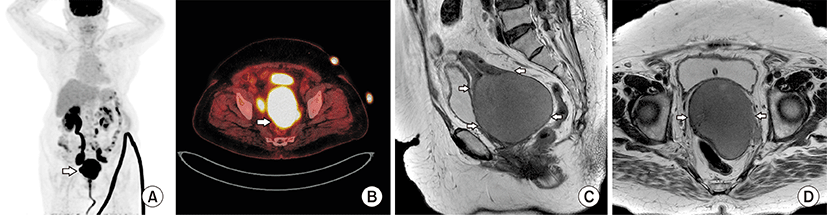
An 84-year-old female patient with medical history of hypertension and diabetes mellitus, without cancer history was referred to our outpatient clinic from local clinic due to symptoms of vaginal bleeding, difficulty in urination as well as abnormal pap smear result of atypical squamous cells of undetermined significance. Upon speculum examination, round mass with smooth and sleek surface without ulceration that resembled cervical leiomyoma was observed. Abdomen and pelvis CT revealed large cervical mass of size 8 cm, with right hydroureterosis, and suspicious metastatic lymphadenopathy at paraaortic, aortocaval, both common iliac, internal iliac and external iliac area, suggestive of cervical cancer. PET-CT also revealed hypermetabolic mass involving whole uterus with left parametrium, with possible metastatic left supraclavicular metastatic lymph nodes (Fig. 1A, B). In pelvis MRI, the mass was seen as low signal intensity in T2-weighted image, and high signal intensity in diffusion weighted imaging (Fig. 1C, D). In laboratory test, hemoglobin was 11.1 g/dL, leukocyte 5,510/μL, platelet 250,000/μL, all within the normal ranges. The tumor markers squamous cell carcinoma antigen was 1.2 ng/mL, CA 125 was 6.9 U/mL, and CEA was 1.5 ng/mL; all within the normal range. HPV PCR test was also negative for high-risk HPV genotypes.
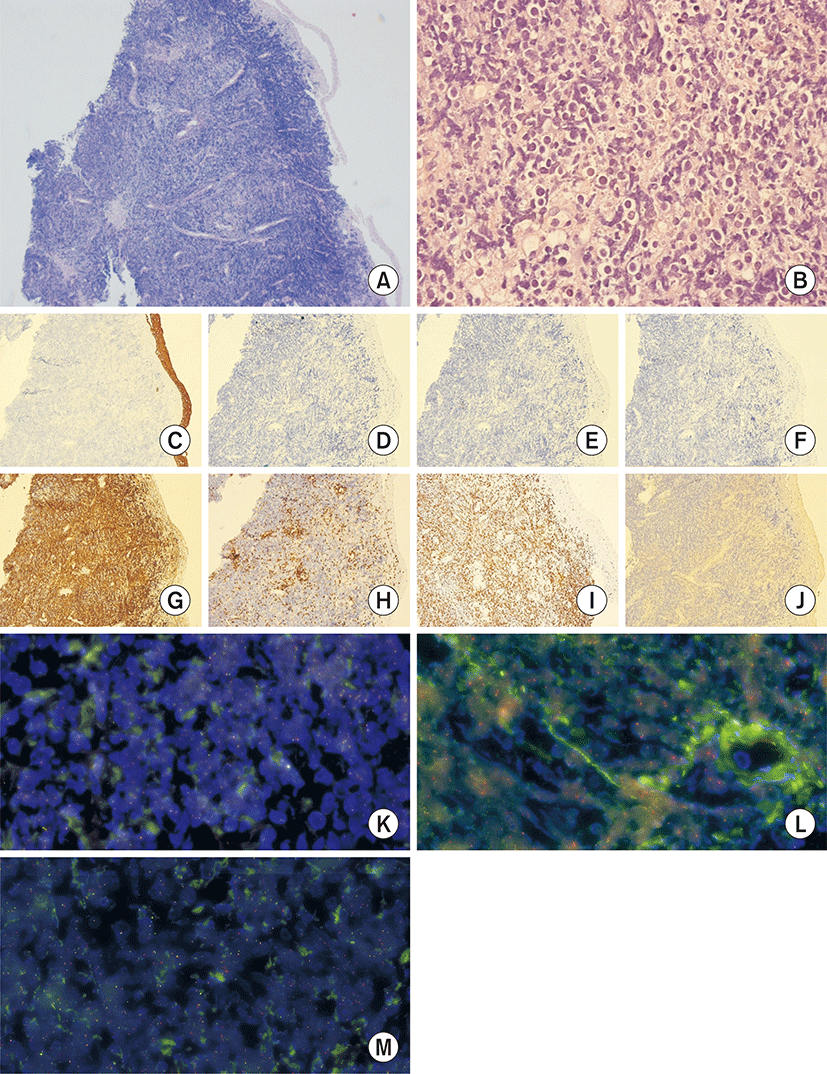
Punch biopsy of the cervix was performed, and the pathologic examination showed diffuse proliferation of large-to-intermediate sized atypical lymphoid cells, as well as positive immunohistochemical stain of pan-B-cell antigens, such as CD20 and CD45, which were findings consistent with diffuse large B-cell lymphoma (Fig. 2). The patient was transferred to our hemato-oncologic clinic, and bone marrow needle biopsy was performed. Bone marrow biopsy revealed normal cellular marrow with trilineage hematopoiesis, without any evidence of lymphoma involvement. Also, Epstein-Barr virus (EBV) infection was detected in peripheral blood. The patient’s stage was determined to be IVA according to Ann Arbor staging classification. Additional fluorescence in situ hybridization (FISH) analysis was performed on formalin-fixed, paraffin-embedded tissue using Vysis LSI dual color, break apart probes (Abbott Molecular, Abbott Park, IL, USA) and 200 non-overlapping nuclei were counted. The cutoff value for positivity was 10% for breakapart probe. The FISH analysis revealed C-MYC and BCL-6 translocation to be positive in tumor cells (Fig. 2), suggesting double hit lymphoma with poor prognosis.
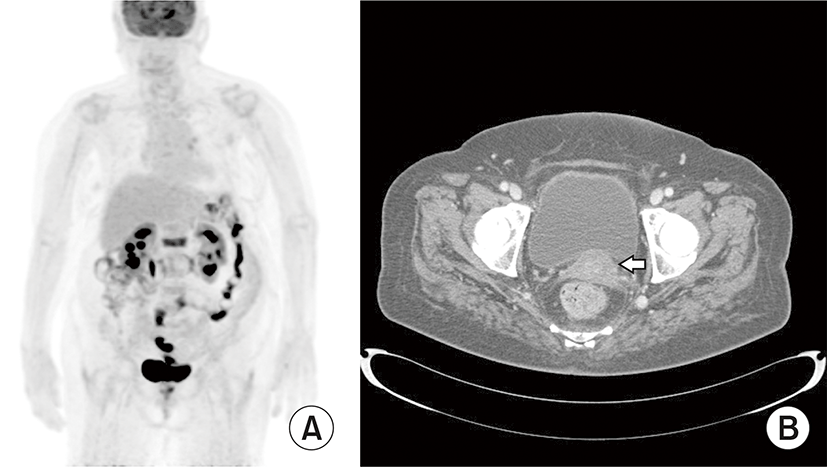
The patient received 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) chemotherapy and achieved partial response. Abdomen and chest CT taken after 6 cycles of chemotherapy revealed markedly decreased state of cervical mass as well as lymph nodes in common hepatic artery, left paraaortic, both common iliac, left external iliac and left supraclavicular area. PET-CT taken at the same time revealed residual viable lymphoma lesion in cervix, but disappeared multiple hypermetabolic lymph nodes in both external iliac, both common iliac, left paraaortic, and left supraclavicular area (Fig. 3). For residual lesions, radiotherapy of 4,140 cGy (180 cGy×23 fractions) was performed, and the patient is currently being followed on outpatient basis every 3 months with suspicious residual disease.
The patient provided written informed consent for the publication and the use of her personal and medical data.
Discussion
NHL can primarily originate from lymph nodes or lymphatic cells located in solid organs [5]. The incidence of NHL is about 20 per 100,000 population, and approximately one third of NHL affects extra-nodal regions [2]. Although most common extra-nodal NHL sites include gastrointestinal tract, skin and central nervous system, other sites such as adrenal gland, breast, thyroid, and female genital tract can also be its origin [2]. Among NHL, diffuse large B-cell lymphoma (DLBCL) accounts for approximately 30%, being the most common histological subtype [6]. The pathomorphological features of DLBCL include intermediate to large cells with large nucleoli and abundant cytoplasm that diffusely infiltrate the architecture of the involved lymph node [6]. Differential diagnosis is made through immunohistochemistry, and markers of B-cell, such as CD20 and CD45, are positive, while T-cell markers are negative. The recommended immunophenotyping panel include CD20, CD3, CD5, CD45, BCL-2, BCL-6, Ki-67, IRF4/MUM1, and MYC [6]. In our case, we found expression of CD20, CD45, BCL-2, BCL-6, Ki-67, MUM1, and C-MYC to be positive, and additional FISH analysis revealed C-MYC and BCL-6 translocation positive, suggesting double-hit lymphoma. Double-hit B-cell lymphomas with MYC and BCL-2 or BCL-6 translocations, are known to be aggressive with poor prognosis, and with median overall survival of only 2.4 to 18 months despite intensive treatment [7]. In addition, our patient was EBV positive, a herpes virus with oncoviral and B-cell lymphotrophic characteristics. EBV-positive DLCBL, not otherwise specified, is a separate entity recognized in the revised World Health Organization 2016 classification, which patients tend to be elderly with poorer response and shorter survival with standard combination chemotherapy compared to those with EBV-negative DLBCL [8].
Mainstay of treatment for DLBCL is R-CHOP, and the total number of cycles are dependent on the stage and bulk of tumor [1-3]. The treatment of limited stage disease (Ann Arbor stage I or II) is also R-CHOP, with or without radiation therapy of involved field. Treatment of advanced stage (stage III or IV) is individualized, but same R-CHOP is most preferred, and various novel biologic agents are currently under study [6].
Among the primary lymphoma of female genital tract, ovary was the most common site, cervix was the second, and uterus, vagina and vulva was the least common [2,9]. NHL that involves cervix only accounts for 0.2% to 0.6%, and primary malignant lymphoma is known to represent only 0.008% of all cervical tumors, only further proving the rarity of the disease [1,9].
Primary cervical lymphomas can be defined as 1) lymphomas that originate and is confined to cervix (and its immediately adjacent lymph nodes or contiguous structures), 2) absence of leukemia (in peripheral blood and bone marrow), and 3) absence of further lymphomatous lesions at remote sites after several months [4,10]. Clinical symptoms of primary cervical lymphoma are usually non-specific, ranging from vaginal bleeding, vaginal discharge, pelvic pain, as well as urinary retention [1-15]. In contrast to the systemic lymphomas, ‘B’ symptoms (fever, night sweats, and weight loss) are rarely reported in cervical lymphomas [1,3].
Imaging studies are not definitive, but can be used for preliminary diagnosis, staging, and for follow-up [4]. Initial investigation of female genital tract is often performed with ultrasonography (US), due to its non-invasive nature. But CT and MRI are often needed additionally to fully assess the lesion’s size, character as well as involvement of adjacent structures. Lymphoma may appear in two patterns: nodular or diffuse. Nodular or multinodular lesions are solid, homogenous, and well-defined, that rarely have necrosis, hemorrhage or calcifications. Diffuse diseases usually show uniform infiltration of the parenchyma of the organs, sometimes causing abnormality of the organ contour and producing organomegaly [4]. In US, lymphomas usually appear hypoechoic without posterior acoustic enhancement, with mild vascularization on color Doppler US [4]. In MRI, lymphoma appear isointense or hypointense in T1-weighted image, while hyperintense in T2-weighted images [4,11]. Uninvolvement of cervicouterine mucosa and sparing of uterine junctional zone are few of the most important imaging findings that differentiate other types of cervical carcinoma from cervical lymphoma [11].
Cervical lymphoma is known to arise from the cervical stroma, and since squamous epithelium is initially uninvolved, pap smears are generally non-diagnostic [12,13]. Only 41% of women with primary cervical lymphoma revealed an abnormal cytology, thus deep cervical biopsy is usually required for diagnosis [12,13]. Pathologically, irrespective of primary or secondary nature of the lesion, most common type of lymphoma in the female genital tract was DLBCL, followed by follicular lymphoma and Burkitt lymphoma [2,14].
Due to rarity of the disease, currently no management guideline is established for primary cervical lymphoma. Both the grade and stage of the disease is considered when determining treatment course, but chemotherapy alone, radiotherapy alone, combination of both, with or without surgery are all available strategies [3,15]. R-CHOP chemotherapy, similar to DLBCL originating from other sites, is most commonly used [1-3]. More recent trends lean towards less invasive option of treatment that does not include surgery, which has been showing good results, especially in patients who desire to preserve their fertility [15].
We described an unusual case of a woman with a diffuse large B-cell lymphoma of cervix. Primary lymphoma of cervix is a rare disease, but it is a reasonable differential diagnosis of atypical cervical tumors. Further research is needed in developing optimal management plan of primary lymphoma of the female genital tract.