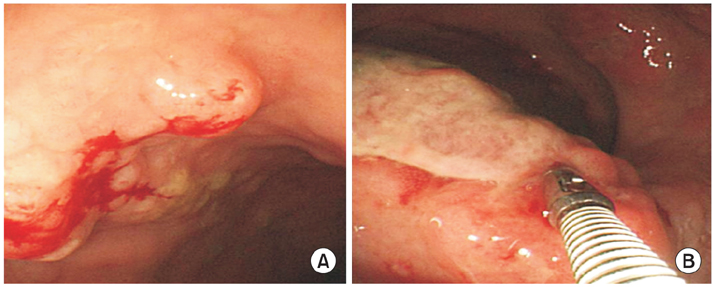
Fig. 1Colonoscopic findings. (A) About 10 mm sized mass-forming lesion with nodularity is observed on rectum. It is diagnosed as mucosa-associated lymphoid tissue lymphoma. (B) About 20 mm sized mass-forming ulcer is observed on rectum, Anal verge (AV) 5 cm. This lesion has vulnerability that doesn't have definite demarcation with surrounding normal mucosa. It is diagnosed as diffuse large B-cell lymphoma.
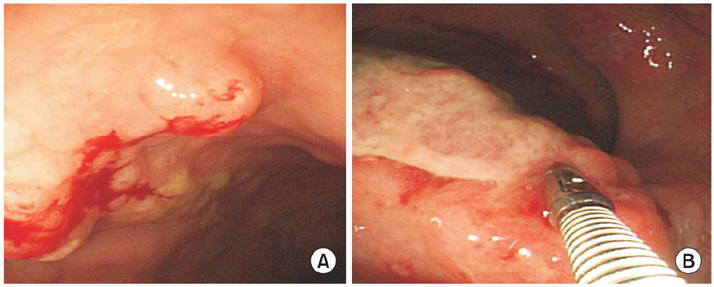
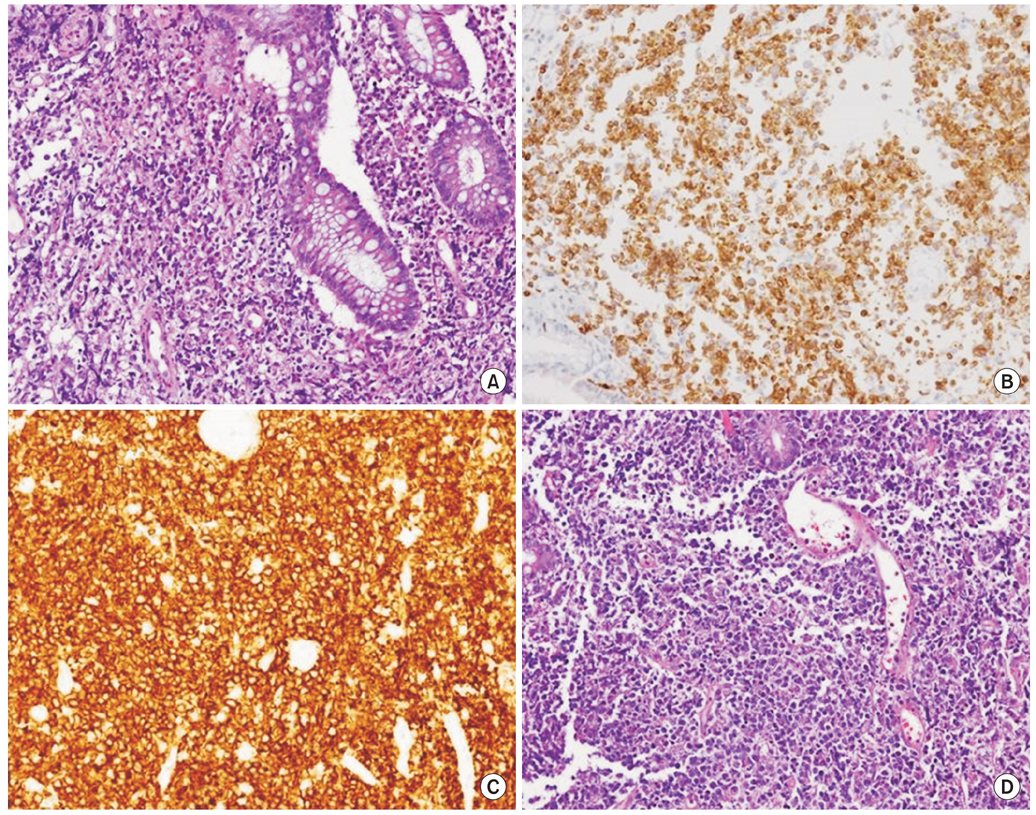
Fig. 2Histopathologic findins of mucosa-associated lymphoid tissue lymphoma (A) and diffuse large B-cell lymphoma (B-D). H&E stain (×200) on rectal biopsy of 20 mm sized mass-forming ulcer shows monomorphic lymphocytic infiltrating of the lamina propria surrounds colonic glands massively infiltrated with atypical lymphocytes and undergoing destruction (lymhpoepithelial lesion) (A). Rectal biopsy specimens of 20 mm sized mass-forming ulcer lesion with nodularity show strong immunoreactivity for BCL 2 stain (×200) (B) and CD 20 stain (×200) (C). (D) H&E stain (×200) shows tumor cells with round or oval nuclei, open chromatin, and prominent nucleoli.
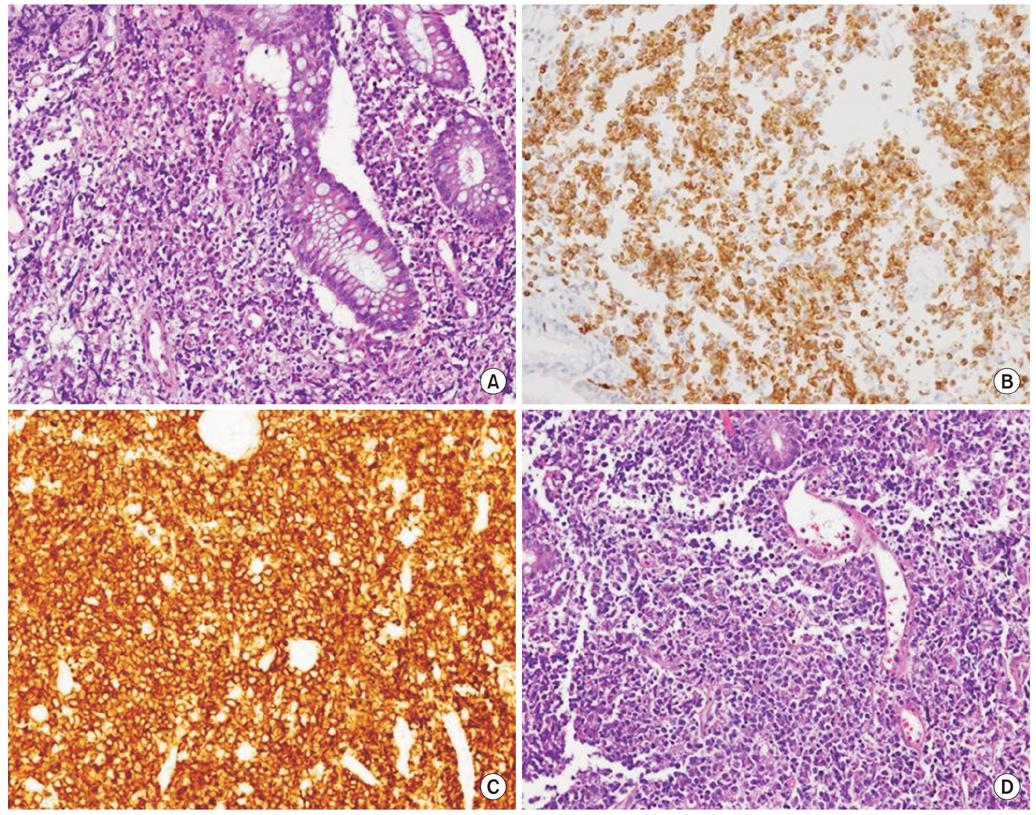
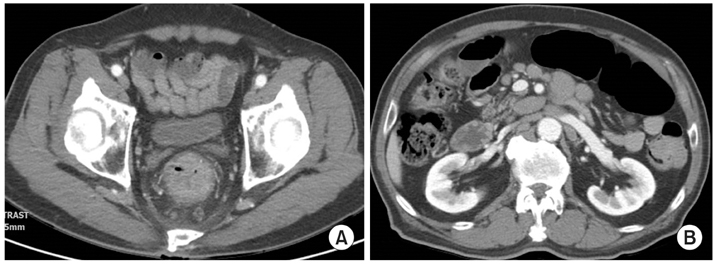
Fig. 3Abdomen and pelvis computed tomography findings at initial presentation. They show circumferential anorectal thickened tissue associated with retro-rectal lymphadenopathy (A) and paraaortic multiple lymphadenpathy (B).
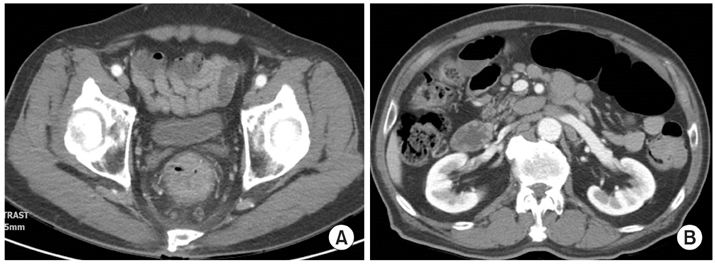
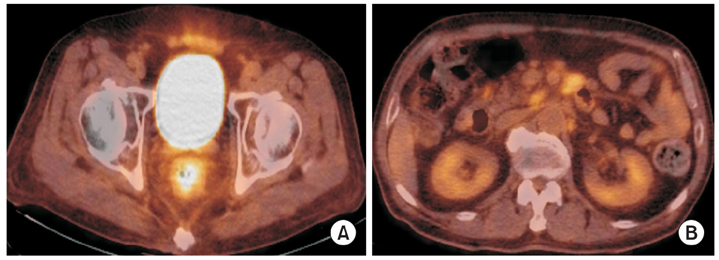
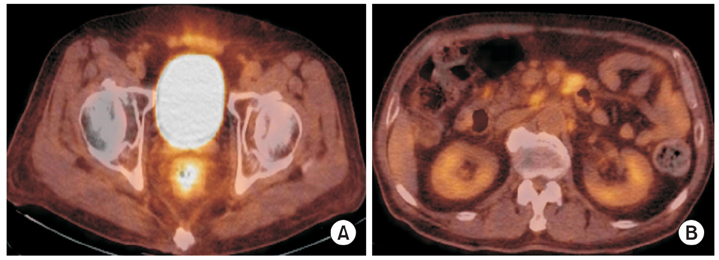
Fig. 4Fluorine-18-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography (18F-FDG PET CT) findings. They show increased FDG uptake in rectal and retroperitoneal paraaortic lymph nodes, which suggest the involvement of lymphoma (A, B).