Introduction
Colorectal cancer (CRC) is the third most common type of cancer worldwide [1]. Peritoneal carcinomatosis (PC) is the second most common cause of death in CRC, following hepatic metastasis [2,3]. Although the development of systemic chemotherapy has improved the survival of metastatic CRC patients, systemic chemotherapy has shown a relatively low drug transmission rate into the peritoneum. Thus, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) was developed for peritoneal malignancies to overcome these limitations. Since colorectal surgical techniques have advanced over several decades [4-6], chemoradiotherapy and extensive lymphadenectomy involving multivisceral resection can be used to treat patients with CRC from the early stages to stage IV [7-9].
Several studies have reported that 4%–15% of CRC patients are diagnosed with either synchronous or metachronous peritoneal metastasis [10]. Franko et al. found that 17.4% of patients with metastatic CRC presented with PC, and for 2.1% of patients, PC was the only metastatic site [11]. Risk factors for peritoneal metastasis include advanced tumor or nodal stages, right-sided tumors, poor differentiation, and an initial emergency procedure for metachronous cancer [12-14]. An analysis of the prognosis of CRC metastatic sites in patients undergoing conventional palliative systemic chemotherapy revealed that patients with peritoneal metastasis had a shorter overall survival than those with other isolated hematogenous metastatic sites [15]. In accordance with the tumor cell entrapment hypothesis proposed by Sugarbaker, surgical manipulation of the primary cancer can lead to locoregional spread of tumor cells. This results in the spillage of free cancer cells, leading to the implantation of these cells on the peritoneal surface, and the exfoliation of numerous cancer cells into the entire peritoneal space [16]. PC progresses rapidly, spreads widely, and induces intestinal obstruction, perforation, or fistula formation, all of which can lead to death.
In this context, CRS combined with HIPEC has been proposed as an alternative treatment option, given that the characteristics of PC differ from those of hematogenous metastasis. However, CRS remains a technically demanding procedure that should be carried out by a highly skilled surgical team to enhance postoperative clinical outcomes. In this study, we have reviewed the fundamental principles of CRS with HIPEC, key considerations, and recent clinical trials concerning the treatment of CRS with HIPEC in CRC patients who have PC.
Preoperative Assessment and Patient Selection
The Peritoneal Cancer Index (PCI) score was developed by Jaquet and Sugarbaker in 1996 [16,17]. They divided the abdominopelvic cavity into nine regions, with four additional segments of the small bowel. The largest tumor lesion is scored from 0 to 3, according to its size, in the respective regions; consequently, the total PCI score ranges from 0 to 39 (3 points×13 regions). The PCI is advantageous for identifying the disease severity, distribution, and location. It is widely known that the PCI score is an important prognostic factor for peritoneal metastatic CRC [18].
The completeness of cytoreduction (CC) score is widely used to evaluate CRS [16]. It consists of four classifications, with each score referring to the remnant tumor burden after CRS (CC-0: no residual tumor, CC-1: <0.25 cm residual tumor, CC-2: 0.25−2.5 cm residual tumor, CC-3: >2.5 cm residual tumor). CC-0 and CC-1 are considered complete cytoreduction, and CC-2 and CC-3 are interpreted as incomplete cytoreduction. A tumor nodule smaller than 0.25 cm is thought to be penetrable by intraperitoneal chemotherapy; thus, CC-1 is regarded as complete cytoreduction if perioperative intraperitoneal chemotherapy is administered. A systematic review found that the median overall survival of complete cytoreduction in patients with PC from CRC was 33 months (range, 20–63 months), while that of patients with incomplete cytoreduction was 8 months (range, 8–17 months) [19]. Complete cytoreduction and a low PCI score are the most important prognostic factors in patients with PC [18].
The Peritoneal Surface Disease Severity Score system consists of the clinical symptom severity, PCI score, and primary tumor histology. The clinical symptoms include weight loss, abdominal pain, and ascites. Symptom severity is defined as follows: no symptoms, mild (weight loss <10% of body weight, mild abdominal pain, and asymptomatic ascites), and severe (weight loss ≥10% of body weight, unremitting pain, bowel obstruction, and symptomatic ascites). The PCI score is divided into three categories (PCI <10, 10–20, >20), and the aggressiveness of the primary tumor histology was classified into three categories (well to moderately differentiated/N0, moderately differentiated/N1 or N2, and poorly differentiated signet ring type). The nine subsections of the Peritoneal Surface Disease Severity Score have their own points, and the total score represents the stage (score 2–3=stage I, score 4–7=stage II, score 8–10=stage III, and score >10=stage IV). This system could be a useful tool for the preoperative prediction of complete resectability in patients with PC [20,21].
Proper patient selection is crucial to avoid surgical morbidity or mortality and to improve the long-term outcomes of patients undergoing CRS and HIPEC. Several preoperative studies for metastatic CRC, including physical examinations; laboratory tests, especially tumor markers; CT of the chest, abdomen, and pelvis; and colonoscopy, should be performed, and additional imaging studies, such as transrectal ultrasonography, liver magnetic resonance imaging, or positron emission tomography-CT, are often performed. Various preoperatively assessable clinicopathological parameters have been evaluated as prognostic markers for CRS and HIPEC.
The best-known established prognostic factors are the PCI and CC scores. Elias et al. retrospectively analyzed 523 patients treated with CRS and intraperitoneal chemotherapy for peritoneal metastatic CRC and found that the PCI score (hazard ratio [HR]=1.052; P<0.001), CC score (HR=1.398; P<0.001), and lymph node invasion (HR=1.534; P<0.02) were associated with poor overall survival [18]. Adjuvant chemotherapy was a significant prognostic factor (HR=0.578; P<0.002). A sub-analysis of this study of 416 patients with CC-0 also found that the presence of liver metastasis and the experience of the center had a significant impact on the long-term prognosis. In a retrospective multicenter study, Glehen et al. reported similar results—namely, complete cytoreduction, treatment with a second procedure, limited extent of PC, age less than 65 years, and adjuvant chemotherapy were identified as positive independent prognostic factors. Preoperative systemic chemotherapy, lymph node invasion, synchronous resection of liver metastasis, and poor tumor differentiation were negative independent prognostic factors [22]. Another study by Elias et al. suggested that small-bowel involvement could be an independent prognostic factor. According to the analysis of 139 patients who had colorectal-origin peritoneal metastasis treated with CRS and HIPEC, a PCI score >15 was always involved in the small bowel, and these patients presented poorer overall survival than patients with a lower PCI score or non-small bowel involvement. Therefore, they proposed that a PCI score of >15 or invasion of the small bowel may be relative contraindications for CRS and HIPEC [23]. In a meta-analysis of the prognostic factors of patients with metastatic CRC who underwent CRS and HIPEC, 25 studies and 10 preoperatively assessable prognostic variables were analyzed and it was found that synchronous liver metastasis, low Eastern Cooperative Oncology Group (ECOG) performance status, lymph node metastasis, poor tumor differentiation, and signet ring cell histology were associated with negative outcomes [24]. Age alone should not be a contraindication for CRS treated with HIPEC, and it is important to select patients based on their performance status, nutritional status, quality of life, and institutional experience [25-27].
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
The aim of CRS is to remove the entire gross tumor burden while avoiding organ dysfunction. A nasogastric tube is inserted within the stomach, and a Foley catheter is placed aseptically after surgical draping to prepare to expose the Foley catheter into the abdominal cavity during the operation [28]. The operative time is commonly longer than that of standard colorectal surgery. Therefore, pneumatic compression devices and prophylactic heparin subcutaneous injection may help prevent deep vein thrombosis [29]. The lithotomy position is routinely used because a pelvic approach or rectal resection is often required. Even in patients who are not expected to undergo pelvic procedures, exploration of the abdominal cavity is crucial for the even distribution of intraperitoneal chemotherapeutic agents for HIPEC. Laparoscopic cytoreduction may be possible when patients have limited metastasis [30]. In patients with relatively high PCI scores or intraperitoneal adhesions, an open approach with a midline incision is preferred for complete CRS. Omentectomy is usually performed by saving the gastroepiploic arteries to avoid delayed gastric emptying. However, if the gastroepiploic arteries are invaded, resection is recommended. The stomach, small bowel, and large bowel, with their mesentery and parietal peritoneum, including the abdominal, subphrenic, and pelvic areas, should be explored and resected if they have tumor invasion. At least 150 cm of the small bowel must be saved to prevent short bowel syndrome. Intraperitoneal organs, including the spleen, gall bladder, uterus, ovaries, and vagina, can be excised during CRS. The feasibility and efficacy of synchronous resection of extraperitoneal metastases, including the liver or lung, in patients with peritoneal metastasis have not been established; however, several reports have demonstrated the feasibility of concurrent liver resection [31,32].
“HIPEC” was recommended as a standard acronym for hyperthermic intraperitoneal chemotherapy by the Fourth International Workshop on Peritoneal Surface Malignancy held in Madrid, Spain in December 2004. The temperature of the intraperitoneal antitumor agent is maintained at 41℃−43℃ with a carrier solution (1.5% or 5% dextrose solution is often used) through a heat exchanger for 30−120 min. The inflow and outflow closed suction catheters are placed in the abdominal cavity and the chemical solution is circulated via a hyperthermia pump.
There are meaningful advantages to using heated cytotoxic drugs administered intraperitoneally. First, low systemic drug levels can be maintained despite high drug concentrations via intraperitoneal administration because of the peritoneal-plasma barrier. When macromolecular anticancer agents are administered into the peritoneum, the anticancer drugs can pass through the peritoneal interstitial layer, but cannot easily pass through the plasma endothelial layer because the gap in the intercellular space of the mesothelium, which composes the peritoneal layer, is wider (0.9 mm) than that of the endothelium (0.5 μm) [33]. It is possible to reduce the side effects of systemic antitumor agents while maximizing their cytotoxic effects on peritoneal tumors. Second, hyperthermia increases drug penetration into the tissues. Antitumor agents penetrate the tumor nodules through passive diffusion, convection, and recirculation [34]. Hyperthermia enhances the drug penetration rate and is expected to lead to a more potent antitumor effect. Third, several chemotherapeutic agents have been reported to exhibit increased cytotoxic effects under hyperthermic conditions. In addition, heat exerts a cytotoxic effect. Hyperthermia can destroy cancer cells not only by inhibiting RNA synthesis, but also by increasing lysosomal activity, which has selective cytotoxic effects on cancer cells [35].
Mitomycin-C (MMC) and oxaliplatin are widely used for HIPEC to treat CRC in patients with PC. They are suitable for intraperitoneal administration with macromolecular drugs (the molecular weights of MMC and oxaliplatin are 334.3 Da and 397.3 Da, respectively) and both are potentiated by hyperthermia. Recommended intraperitoneal regimens include 35 mg/m2 of MMC in an isotonic salt solution for 90 minutes for the first 50% of the dose, followed by 25% dose at 30 and 60 minutes, and 460 mg/m2 of oxaliplatin in 5% dextrose solution for 30 minutes. According to a review article, the number of studies that enrolled more than 100 patients with CRC was approximately 20, and almost all of them used MMC or oxaliplatin (cisplatin and irinotecan are also used, but only in combination with MMC or oxaliplatin) [36]. Several comparative studies have analyzed the long-term outcomes of MMC and oxaliplatin; however, the overall superiority has not been identified yet [37].
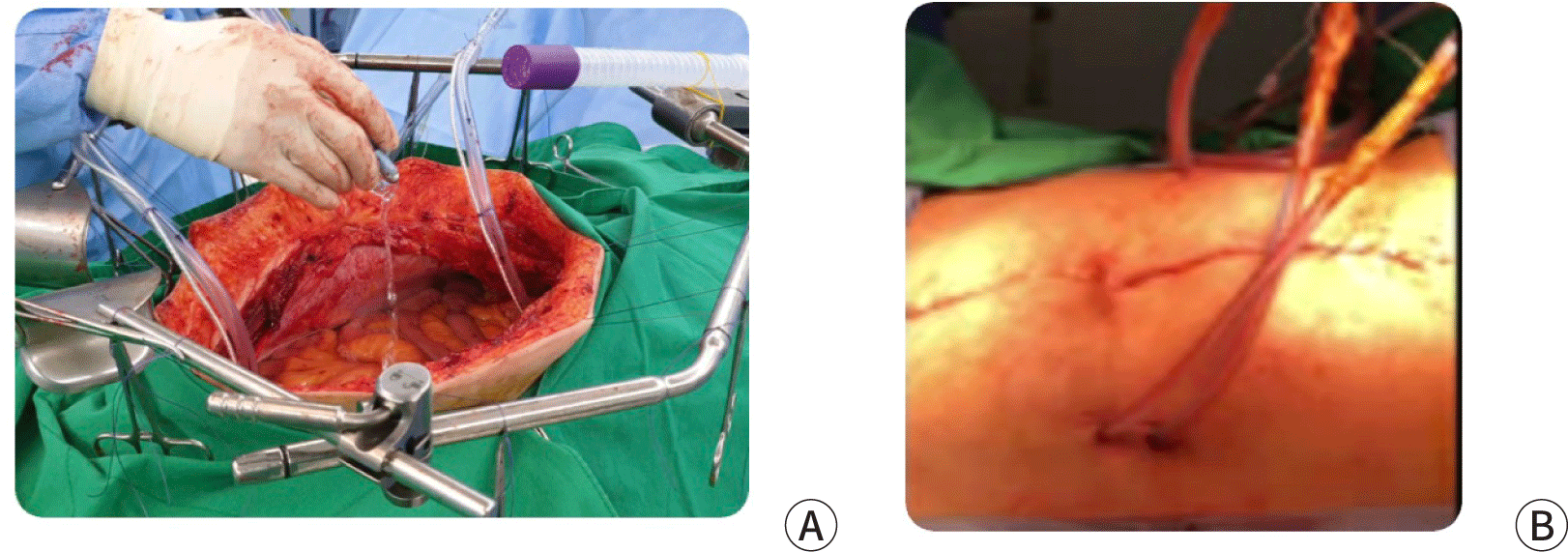
HIPEC techniques are divided into the open coliseum technique and the closed technique (Fig. 1). The open coliseum technique has been previously described by Sugarbaker [16]. The major advantage of the open coliseum technique is that surgeons can distribute chemotherapy solutions manually; therefore, an even temperature and a proper distribution of antitumor agents are maintained. However, surgeons might be confronted with the potential hazard of exposure to the chemotherapy solution in its own form or as an aerosol. In contrast, the closed technique has the advantage of minimizing heat loss. A retrospective study that compared the hemodynamic distinction between open and closed HIPEC techniques showed no significant differences, except for the intraperitoneal temperature (more stable temperature maintenance in the closed technique) [38]. However, a major disadvantage of the closed technique is the non-homogeneous distribution of the chemotherapy solution and temperature. This may lead to uneven treatment effects in the intraperitoneal cavity or morbidity due to the overheated solution. Surgeons should consider the advantages and disadvantages of the two HIPEC techniques when choosing the method.
The morbidity of CRS and HIPEC is associated with postoperative surgery-related complications, including anastomotic leakage, wound infection, intra-abdominal sepsis, intestinal obstruction, and bleeding. Additionally, intraperitoneal chemotherapy-related complications, such as neutropenia, renal toxicity, and arrhythmia may occur. According to a systematic review of the efficacy of CRS and HIPEC for PC in CRC, the postoperative overall morbidity rates ranged from 23% to 44%, mortality rates ranged from 0% to 12%, and reoperation rates ranged from 4% to 11% based on nine studies [39]. Intraperitoneal MMC-induced neutropenia is a frequent complication that has been reported to occur in 39% to 40% of patients [40]. Lambert et al. reported an association between female sex and MMC-HIPEC-induced neutropenia, and they speculated that female patients have a larger peritoneal surface area with a smaller plasma volume than male patients, which may affect the pharmacological effect of MMC [40].
Recent Clinical Trials for Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy
A randomized controlled trial compared CRC patients who received systemic chemotherapy using fluorouracil and leucovorin (n=51) and underwent CRS/HIPEC with or without adjuvant chemotherapy (n=54) from 1998 to 2001 in the Netherlands [2]. In this trial, HIPEC was performed using 35 mg/m2 of MMC by a triple method over 90 minutes. The median overall survival was 22.3 months in the CRS/HIPEC group and 21.6 months in the CRS/HIPEC and systemic chemotherapy groups, respectively (P=0.032). Although this trial has some limitations due to its inclusion of patients with CRC and appendiceal neoplasms, it is the first randomized controlled trial to show a survival benefit of CRS/HIPEC compared with systemic chemotherapy only in CRC patients with peritoneal metastases.
The PRODIGE-7 trial was performed with 256 enrolled patients at 17 French centers from 2008 to 2014 [41]. The CRC patients with peritoneal metastases were randomly assigned to the CRS group (n=132) or the CRS/HIPEC group (n=133) by 1:1 allocation. HIPEC was intravenously administered using oxaliplatin (460 mg/m2) mixed in a 5% dextrose carrier solution with bidirectional chemotherapy with folinic acid (20 mg/m2) and 5-fluorouracil (400 mg/m2) intravenously. In this study, the median survival was 41.7 months in the CRS/HIPEC group and 41.2 months in the CRS-only group (P=0.995). The 1-year survival rates in the CRS/HIPEC and CRS-only groups were 86.9% and 88.3%, respectively. The 5-year survival rates were 39.4% and 36.7% in the CRS/HIPEC and CRS-only groups, respectively. The relapse-free survival in the CRS/HIPEC group was 13.1 months, while that in the CRS-only group was 11.1 months (P=0.486). Thus, there was no significant difference in overall survival or relapse-free survival between the CRS/HIPEC and CRS-only groups. However, the hospital stay was longer in the CRS/HIPEC group than in the CRS-only group (18 vs. 13 days, P=0.0001). The rate of grade III postoperative adverse events was 26% in the CRS/HIPEC group, which was higher than that in the CRS group (15%; P=0.035). Therefore, the PRODIGE 7 trial concluded that no survival benefits were achieved by adding HIPEC after CRS in patients with CRC with peritoneal metastases.
Although the PRODIGE 7 trial failed to show a survival benefit from the addition of HIPEC to the treatment of CRC with peritoneal metastases, its results have been criticized. First, the prolonged overall survival in both the CRS and CRS/HIPEC groups was remarkable compared with the survival after palliative systemic chemotherapy. The overall survival of CRC with peritoneal metastasis in an analysis of the ARCAD database was 16.3 months, while the CRS and CRS/HIPEC groups showed overall survival of 41.2 months and 41.7 months, respectively [42]. Thus, the importance of surgical resection to reduce the tumor burden should be acknowledged when interpreting the results of the PRODIGE 7 trial [43]. In addition, the pharmacologic drawbacks of oxaliplatin with HIPEC are also criticized regarding the interpretation of the trial results. Specifically, the short half-life and rapid absorption of oxaliplatin into the plasma mean that it is not a suitable agent for increasing the efficacy of intraperitoneal chemotherapy. In addition, the carrier solution using 5% dextrose solution has disadvantages for use in the peritoneal cavity due to the influence of high glucose levels and delayed hemorrhagic complications [44]. Thus, there has been a trend to select MMC instead of oxaliplatin for the HIPEC regimen in CRC patients after CRS after the results of the PRODIGE 7 trial [45]. Nonetheless, the role of HIPEC and the appropriate chemotherapeutic agents in CRC patients are still debated.
The PROPHYLOCHIP-PRODIGE 15 trial was a randomized phase III trial to evaluate second-look surgery with HIPEC in CRC patients at high risk for peritoneal metastases compared with surveillance [46]. Patients who had synchronous or localized peritoneal seeding during primary tumor resection, a perforated tumor, or surgical removal of ovarian metastases were considered to have a high risk of peritoneal recurrence. Thus, 150 patients from 23 hospitals in France were randomly assigned to the second-look surgery group with HIPEC (oxaliplatin 460 mg/m2 or oxaliplatin 300 mg/m2 with irinotecan 200 mg/m2 plus 5-fluorouracil 400 mg/m2 intravenously or MMC 35 mg/m2) or the surveillance group between 2010 and 2015. Second-look surgery was performed after 6 months of adjuvant chemotherapy with no signs of recurrence and continued to CRS/HIPEC when there was evidence of peritoneal recurrence during surgery. Interestingly, 71 patients experienced peritoneal recurrence in the surveillance group (48%) and the second-look surgery group (47%). The most common site of recurrence was the peritoneum, followed by the liver. However, there was no significant difference in overall survival and disease-free survival between the surveillance and second-look surgery groups with or without CRS/HIPEC. The 5-year overall survival rate was 72% in the surveillance group, which was not significantly different from that of 68% in the second-look surgery group. The 5-year disease-free survival rate was also not significantly different between the surveillance and second-look arthroscopy groups (49% and 42%, respectively; P=0.82). Therefore, this study showed no improvement in the outcomes of second-look surgery with HIPEC compared with surveillance. However, it is remarkable that this study suggested that peritoneal metastasis developed during the treatment of CRC patients with a high risk of recurrence, although radiologic results showed no evidence of recurrence.
The COLOPEC randomized multicenter trial aimed to evaluate the role of adjuvant HIPEC in preventing peritoneal metastases in CRC patients with a high risk of peritoneal recurrence [47]. A high risk of peritoneal recurrence was defined as T4N0-2M0 on the preoperative findings or pathologic T4 stages or a perforated primary tumor. In this study, the patients were stratified by tumor characteristics (T4 or perforation), age (≤65 years of >65 years), and the surgical approach for primary tumor resection (laparoscopy or open surgery). From 2015 to 2016, 204 patients were randomly assigned to either the adjuvant systemic chemotherapy group (n=102) or the adjuvant HIPEC with systemic chemotherapy group (n=102). In this study, HIPEC was performed using oxaliplatin (460 mg/m2) at 42℃–43℃ for 30 minutes with bidirectional 5-fluorouracil (400 mg/m2) and leucovorin (20 mg/m2). The overall survival of the systemic chemotherapy group and 94.1% in the adjuvant HIPEC group was 93% (P=0.82) and disease-free survival of the systemic chemotherapy group and 69.3%, in the adjuvant HIPEC group was 69% (P=0.99). Thus, the COLOPEC trial did not show any benefits of adjuvant HIPEC in patients with T4 or perforated primary tumors.
The HIPECT 4 trial also aimed to evaluate the role of HIPEC in the prevention of peritoneal recurrence in patients with T4N0-2M0 CRC [48]. This study was designed to randomly allocate patients to a surgery with systemic chemotherapy group and a surgery with systemic chemotherapy and HIPEC (MMC 30 mg/m2, 60 min, 42℃–43℃) group. The primary endpoint was locoregional control survival, and the secondary endpoints were perioperative morbidity/mortality, overall survival, and disease-free survival. Between 2015 and 2021, 184 patients at 17 Spanish centers were allocated to the surgery-alone group (n=95) and the surgery and HIPEC group (n=89). In this study, the 3-year locoregional control rate of surgery in the HIPEC group was 97.6%, which was significantly higher than that in the control group (87.6%, P=0.03); however, there was no significant difference in disease-free survival or overall survival between the two groups. There were no significant differences in adverse toxic events or morbidities between the groups. Notably, the HIPECT 4 trial used MMC as the chemotherapeutic regimen for HIPEC, whereas the COLOPEC and PROCHYLOCHIP trials used oxaliplatin. Although the HIPECT 4 trial showed the advantages of local control using MMC HIPEC, future studies are needed to determine the role of HIPEC in the prevention of recurrence in CRC patients with a high risk of peritoneal metastases (Table 1).
| Trials | Enrollment period | Country | Published | Control vs. experimental arm | HIPEC (drug, dose) | Inclusion criteria |
|---|---|---|---|---|---|---|
| Netherland trial [2] | 1998−2001 | Netherlands | 2003 | Systemic CTx (n=51) vs. CRS/HIPEC+adjuvant CTx (n=54) | Mitomycin-C 35 mg/m2 | CRC PM |
| PRODIGE-7 [41] | 2008−2014 | France | 2021 | CRS (n=132) vs. CRS/HIPEC (n=133) followed by adjuvant CTx | Oxaliplatin 360−460 mg/m2 +IV 5-FU/LV | CRC PM, PCI<26 |
| ProphyloCHIP [46] | 2010−2015 | France | 2020 | Surveillance vs. Second-look surgery+HIPEC | Oxaliplatin 360−460 mg/m2 +IV 5-FU/LV or Mitomycin-C 35 mg/m2 | Patients with resected synchronized localized CRC PM or perforated tumor |
| COLOPEC [47] | 2015−2017 | Netherlands | 2019 | Adjuvant CTx vs. Adjuvant HIPEC+adjuvant CTx | Oxaliplatin 360−460 mg/m2 +IV 5-FU/LV | Resected T4N0-2M0 or perforated CRC |
| HIPECT4 [48] | 2018−2021 | Spain | 2023 | Adjuvant CTx vs. Adjuvant CTx+HIPEC | Mitomycin-C 30 mg/m2, 60 min | Resected cT4NxMx CRC |
The current clinical trials for CRS/HIPEC have several issues regarding the selection of appropriate chemotherapeutic agents that are modified adequately for use in peritoneal chemotherapy. Careful patient selection to increase the efficacy of CRS/HIPEC is also important for improving the oncological outcomes. The development of intraperitoneal chemotherapeutic agents and genetic analyses based on individual tumor characteristics are needed to improve the oncologic outcomes after CRS/HIPEC.
Conclusion
CRC with peritoneal metastasis has a poor prognosis, underscoring the need to overcome the limitations of current treatments. Thus, CRS/HIPEC can be regarded as a treatment option for improving survival. Cytoreduction is thought to improve survival by reducing the tumor burden in patients with stage IV CRC. In addition, the principles and pharmacological characteristics of intraperitoneal chemotherapy have advantages over systemic chemotherapy. A more precise diagnosis, improved HIPEC techniques, and careful patient selection are needed for treatment with CRS/HIPEC. Ongoing clinical trials are expected to highlight the roles of CRS and HIPEC in patients with CRC and peritoneal metastases.

