
 , Minsung Kim
, Minsung Kim
Enhanced recovery after surgery (ERAS) protocols are designed to minimize surgical stress, preserve physiological function, and expedite recovery through standardized perioperative care for primary colorectal surgery patients. This narrative review explores the benefits of current ERAS protocols in improving outcomes for these patients and provides insights into future advancements. Numerous studies have shown that ERAS protocols significantly reduce the length of hospital stays by several days compared to conventional care. Additionally, the implementation of ERAS is linked to a reduction in postoperative complications, including lower incidences of surgical site infections, anastomotic leaks, and postoperative ileus. Patients adhering to ERAS protocols also benefit from quicker gastrointestinal recovery, marked by an earlier return of bowel function. Some research indicates that colorectal cancer patients undergoing surgery with ERAS protocols may experience improved overall survival rates. High compliance with ERAS protocols leads to better outcomes, yet achieving full adherence continues to be a challenge. Despite these advantages, implementation challenges persist, with compliance rates affected by varying clinical practices and resource availability. However, the future of ERAS looks promising with the incorporation of prehabilitation strategies and technologies such as wearable devices and telemedicine. These innovations provide real-time monitoring, enhance patient engagement, and improve postoperative follow-up, potentially transforming perioperative care in colorectal surgery and offering new avenues for enhanced patient outcomes.
Citations

Over the past 3 years, the COVID-19 pandemic has posed significant challenges to the healthcare system, leading to delays in the diagnosis and treatment of various diseases due to the need for social distancing measures. Colorectal cancer has not been immune to these disruptions, and research in various countries has explored the impact of COVID-19 on the diagnosis and treatment of colorectal cancer. One notable consequence has been the postponement of colorectal cancer screenings, potentially resulting in disease progression, which can adversely affect surgical and oncological outcomes. Furthermore, the treatment approach for colorectal cancer may vary depending on the extent of disease progression and the healthcare policies implemented in response to the COVID-19 pandemic. In this systematic review, we examine treatment strategies, surgical outcomes, and oncological variables across multiple studies focusing on colorectal cancer treatment during the COVID-19 pandemic. The purpose of this analysis was to assess how medical policies enacted in response to the COVID-19 pandemic have influenced the outcomes of colorectal cancer treatment. We hope that this review will provide valuable insights and serve as a foundational resource for developing guidelines to address potential medical crises in the future.
 , Eun Jung Park
, Eun Jung Park
In stage IV colorectal cancer (CRC), peritoneal metastasis is associated with a poor prognosis. Hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery (CRS) is an effective treatment option that offers survival benefits in patients with peritoneal metastatic CRC. For over the past several decades, a multitude of studies have been conducted on CRS and HIPEC for peritoneal metastatic diseases, and research in this area is ongoing. Proper patient selection and a meticulous preoperative assessment are crucial for achieving successful postoperative outcomes. The completeness of cytoreduction and the surgical techniques employed are key factors in improving oncologic outcomes following CRS and HIPEC. The role of HIPEC for both therapeutic and prophylactic purposes is currently being evaluated in recent clinical trials. This article reviews the fundamental principles of CRS combined with HIPEC and discusses recent clinical trials concerning the treatment of CRS and HIPEC in CRC patients with peritoneal carcinomatosis.

The primary objective in the treatment of early rectal cancer is to achieve optimal oncological control while minimizing the long-term impact of therapeutic interventions on patients' quality of life. The current standard of care for most stage I and II rectal cancers involves radical surgery, specifically total mesorectal excision. Although total mesorectal excision is generally curative for early rectal cancers, it can significantly affect patients' quality of life by potentially necessitating a permanent colostomy and causing bowel, bladder, and sexual dysfunction. Given the morbidity associated with radical surgery, alternative approaches to managing early rectal cancer, such as local excision through transanal excision, transanal endoscopic microsurgery, and transanal minimally invasive surgery, have been investigated. If these surgical approaches are applied cautiously to carefully selected cases of early rectal cancer, it is anticipated that these local procedures will achieve comparable oncological outcomes to the established standard of radical surgery, potentially offering superior results regarding morbidity, mortality, and overall quality of life.

Preoperative chemoradiotherapy (pCRT) followed by total mesorectal excision is the accepted standard treatment for patients with locally advanced rectal cancer. The purpose of pCRT is to prevent the spread of viable tumor cells within the local area during surgical procedures. Additionally, pCRT can facilitate the resection of locally advanced tumors that are otherwise challenging to remove, thereby enabling a radical resection. Although a pathologic complete response is observed in fewer than 20% of patients, the reasons for the variability in tumor response to pCRT are not fully understood. Several techniques have been researched with the aim of improving the tumor response to pCRT. These techniques include intensifying or combining chemotherapy, either simultaneously or sequentially, increasing radiation dose, modifying radiation mode or schedule, adjusting the interval between radiation and surgery, and incorporating multiple agents to increase the efficacy of pCRT. This review discusses various strategies that may improve tumor response outcomes following pCRT.
 , Il Tae Son
, Il Tae Son , Bo Young Oh
, Bo Young Oh
Colorectal cancer (CRC) is a globally prevalent and challenging malignancy. Accurate prognosis prediction is essential for optimizing patient care. This comprehensive review discusses the intricate relationships between inflammatory response markers and CRC prognosis. Inflammatory response markers have gained prominence as a prognostic tool. Elevations in the preoperative neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and C-reactive protein-albumin ratio predict a poor prognosis for patients with CRC. A decreased lymphocyte-monocyte ratio is also a poor prognostic factor. A high Glasgow prognostic score and a high modified Glasgow prognostic score are associated with adverse outcomes, including reduced survival. While significant progress has been made, challenges remain in standardizing the clinical application of these inflammatory response markers. Prospective research and further investigations are warranted to refine the prognostic models. Enhanced understanding and utilization of these inflammatory response markers will help advance personalized treatment strategies, refine surveillance protocols, and improve the management of CRC.
Citations


The rate of colorectal cancer (CRC) has altered. Early-onset CRC patients are increasing, and it is one of the main causes of cancer-related death. Based on epidemiologic change, the CRC screening program needs to be changed. To increase compliance, non-invasive screening techniques are developed. Although CRC survival has increased, the oncologic prognosis of metastatic CRC is remains poor. Even in metastatic CRC, which is the most difficult to treat, attempts are being made to increase the survival rate by active surgical therapy with the creation of chemotherapeutic regimens and targeted treatment based on genomic information. Due to the introduction of aggressive chemotherapy regimens, targeted therapy based on genomic features, and improvements in surgical technique, the role of surgical treatment in metastatic CRC has expanded. Metastatic CRC surgery was indicated for liver, lung, and even peritoneal seeding. Local ablation therapy was also effectively used for liver and lung metastasis. Cytoreductive surgery and intraperitoneal chemotherapy were tried for peritoneal seeding and demonstrated good results in a subgroup of patients, although the right indication was carefully assessed. At the same time, one of the key goals of treatment for CRC was to maintain functional outcomes. Neoadjuvant treatment, in particular, helped rectal cancer patients preserve functional results while maintaining oncologic safety. Rectal cancer organ preservation techniques are now being researched heavily in a variety of neoadjuvant treatment settings, including immunotherapy and whole neoadjuvant therapy. Precision medicine based on patient and disease characteristics is currently being used for the diagnosis and treatment of CRC.
Citations


Low anterior resection syndrome (LARS) is a condition of anorectal dysfunction that occurs frequently following anal sphincter-preserving surgery for rectal cancer and can reduce the quality of life. In this review, we summarize the main symptoms and pathophysiology of this syndrome and discuss the treatment approaches. Early evaluation and initiation of appropriate treatment postoperatively are crucial. The most frequently used tool to evaluate the severity of LARS is the LARS score, and an anorectal manometer is used for objective evaluation. LARS is believed to be caused by multiple factors, and some of its causes include direct structural damage to the anal sphincter, damage to the innervation, loss of rectoanal inhibitory reflex, and decreased rectal volume and compliance. Diet modifications, medications, pelvic floor muscle training and biofeedback are the primary treatments, and rectal irrigation can be added as a secondary treatment. If LARS symptoms persist even after 1 to 2 years and significantly reduce the quality of life, antegrade irrigation, sacral nerve stimulation or definitive stoma may be considered. High-quality evidence-based studies on LARS treatment are lacking, and randomized controlled trials aimed at developing severity-based treatment algorithms are needed.
Citations

Minimally invasive surgery for colorectal disease has now become the standard treatment in Republic of Korea. However, there are limitations to the laparoscopic approach, such as an unstable camera support, a limited range of motion, and poor ergonomics. Recent advances in technology have led to the introduction of robotic surgical systems in colorectal surgery to overcome these shortcomings. Robot-assisted colorectal surgery has clear advantages in many aspects. Surgery involving the rectum benefits the most among colorectal diseases owing to technical difficulties in rectum dissection. The concept of robotic surgery is not different from laparoscopic surgery in that it is a minimally invasive surgery, and abundant research demonstrates comparable results from both modalities for postoperative complications, oncological outcomes, and functional outcomes. However, the cost of robot-assisted surgery limits surgeons to performing robotic surgeries in only selected cases. Improvements regarding cost-effectiveness and more convincing studies that support benefits of robotic surgery are needed to popularize robot-assisted colorectal surgery.
Citations


Local recurrence was reduced considerably due to the introduction of neoadjuvant chemoradiotherapy as treatment for locally advanced rectal cancer. However, certain proportions of patients would experience local recurrence inevitably; the lateral pelvic lymph node is the primary site of rectal cancer recurrence even after administering neoadjuvant chemoradiotherapy. It remains unknown whether lateral pelvic lymph node metastasis is considered as a locoregional disease or a distant metastasis. Although the oncologic stance of lateral pelvic lymph node metastasis is controversial, there is increasing research interest in evaluating the conditional benefit of lateral pelvic lymph node dissection in a subgroup of patients. Researchers reported an improvement in local control in patients with clinically suspected lateral pelvic lymph node metastasis before/or after neoadjuvant chemoradiotherapy who underwent lateral pelvic lymph node dissection. However, there is no clear consensus regarding the indication, diagnostic method, and extent of lateral pelvic lymph node dissection.
Citations

Over the past decade, substantial advances have been made in the individualization of therapeutic strategies for metastatic colorectal cancer (mCRC). Treatment strategies have been developed and classified according to their molecular and genetic characteristics based on predictive biomarkers such as microsatellite instability,
Citations

 , Kwang Ho Kim
, Kwang Ho Kim , Soon Sup Chung
, Soon Sup Chung , Kyoung Sook Hong
, Kyoung Sook Hong , Ryung-Ah Lee
, Ryung-Ah Lee
In the metastatic process, interactions between circulating tumor cells (CTCs) and the extracellular matrix or surrounding cells are required. β1-integrin may mediate these interactions. The aim of this study was to investigate whether β1-integrin is associated with the detection of CTCs in colorectal cancer.
We enrolled 30 patients with colorectal cancer (experimental group) and 30 patients with benign diseases (control group). Blood samples were obtained from each group, carcinoembryonic antigen (CEA) mRNA for CTCs marker and β1-integrin mRNA levels were estimated by using reverse transcription-polymerase chain reaction, and the results were compared between the two groups.
CEA mRNA was detected more frequently in colorectal cancer patients than in control patients (P=0.008). CEA mRNA was significantly reduced after surgery in the colorectal cancer patients (P=0.032). β1-integrin mRNA was detected more in colorectal cancer patients than in the patients with benign diseases (P<0.001). In colorectal cancer patients, expression of β1-integrin mRNA was detected more for advanced-stage cancer than for early-stage cancer (P=0.033) and was significantly decreased after surgery (P<0.001). In addition, expression of β1-integrin mRNA was significantly associated with that of CEA mRNA in colorectal cancer patients (P=0.001).
In conclusion, β1-integrin is a potential prognostic factor following surgical resection in colorectal cancer patients. β1-integrin may be a candidate for use as a marker for early detection of micrometastatic tumor cells and for monitoring the therapeutic response in colorectal cancer patients.

Hereditary nonpolyposis colorectal cancer (HNPCC) is the most common hereditary colorectal cancer syndrome and accounts for about 5% of colorectal cancer. It is inherited as autosomal dominant type and is caused by germline mutations in mismatch repair genes such as
 , Hyun Joo Song
, Hyun Joo Song , Min Jung Kim
, Min Jung Kim , Weon Young Chang
, Weon Young Chang , Bong Soo Kim
, Bong Soo Kim , Chang Lim Hyun
, Chang Lim Hyun
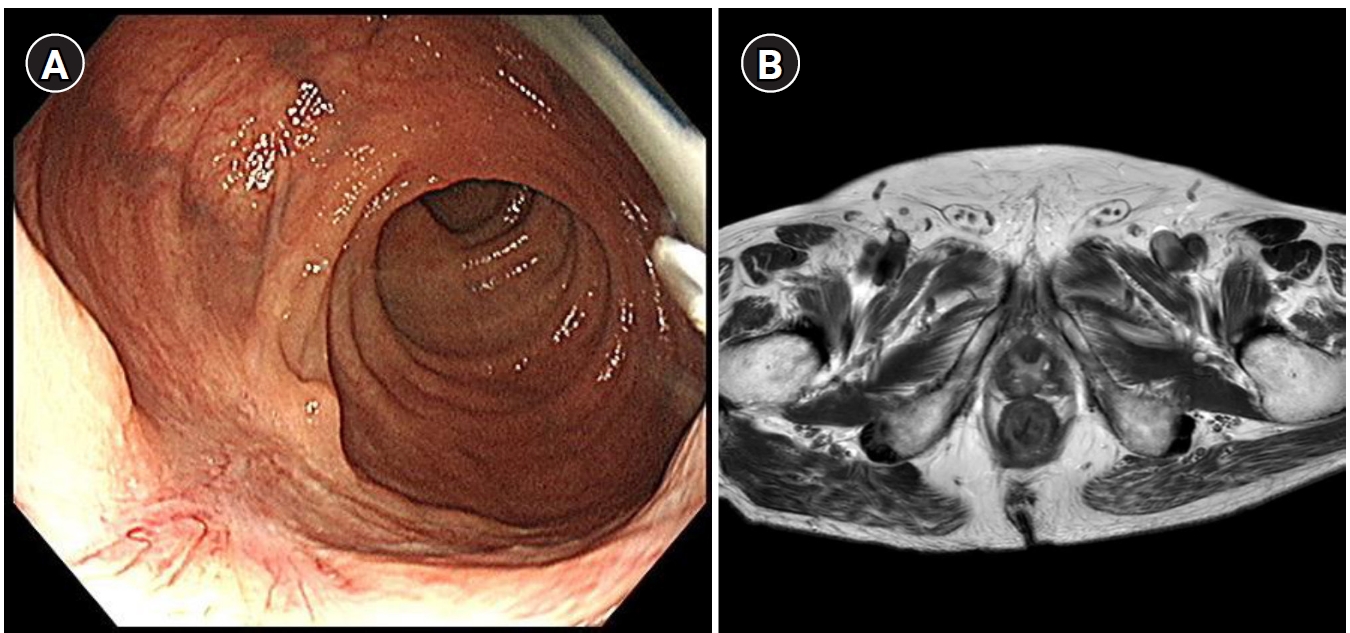
Solitary rectal ulcer syndrome (SRUS) is a rare benign and chronic rectal disease that has a wide spectrum of clinical presentations and variable endoscopic findings. It is usually diagnosed by histopathological examination through biopsy. A 68-year-old man was referred to our hospital with anal pain and difficulty on bowel movement. Colonoscopy showed a hemorrhagic ulcerated mass in the rectum. All radiologic findings such as abdominopelvic computed tomography (CT), positron emission tomography-CT and magnetic resonance imaging were suspicious of rectal cancer. Although the patient underwent repeat endoscopic biopsy and one surgical biopsy, the results were not indicative of malignancy. Two months after conservative management, clinical symptoms and colonoscopic findings were markedly improved. Thus, we report this rare case of a 68-year-old man who had a central ulcerated mass that mimicked rectal cancer on gross colonoscopic and radiologic findings, representing an SRUS variant.
Citations


