Abstract
Non‑operative management, particularly the watch and wait (WW) strategy, has emerged as an alternative to total mesorectal excision for selected patients with locally advanced rectal cancer who achieve a clinical complete response (cCR) after neoadjuvant treatment. This narrative review examines oncologic outcomes, functional and quality‑of‑life benefits, diagnostic challenges, and surveillance requirements associated with WW compared to radical surgery. Evidence from randomized trials and international registries indicates that WW provides overall and disease-free survival rates comparable to those of surgery, provided that stringent selection criteria and intensive surveillance are maintained for 3 to 5 years. Local regrowth occurs in 15%–40% of patients—most commonly within 24 months—but salvage surgery is curative in over 90% of cases and restores oncologic equivalence. Nevertheless, distant metastasis is more frequent in patients who experience regrowth, underscoring the importance of early detection and the need for optimized systemic therapy. Accurate determination of cCR remains the primary limitation; digital rectal examination, high‑resolution magnetic resonance imaging, and endoscopy, even when combined, cannot reliably exclude microscopic residual disease. Total neoadjuvant therapy increases cCR rates to 30%–60% and expands the pool of WW candidates, but also intensifies the need for standardized response definitions and surveillance algorithms. WW offers organ preservation and quality‑of‑life improvements without compromising survival in carefully selected patients, provided that multidisciplinary teams ensure rigorous response assessment and lifelong monitoring. Future advances in imaging, molecular biomarkers, and individualized risk stratification are expected to further enhance the safety of WW and expand eligibility to a broader patient population.
-
Keywords: Biomarkers; Disease-free survival; Neoadjuvant therapy; Rectal neoplasms; Quality of life
Introduction
Background
The management of locally advanced rectal cancer (LARC) has evolved markedly in recent decades, shifting from predominantly aggressive surgical interventions to more integrated, multidisciplinary approaches. Neoadjuvant chemoradiotherapy (nCRT), followed by total mesorectal excision (TME) and adjuvant chemotherapy, has become the standard treatment for LARC [
1,
2]. This approach has substantially improved local disease control and increased rates of sphincter preservation compared to surgery alone. However, radical surgery for rectal cancer can severely impair functional outcomes and is often associated with diminished quality of life [
2]. Moreover, despite advances in local tumor control, these strategies have not consistently yielded improvements in overall survival, prompting a reassessment of treatment intensity and its attendant morbidity [
1-
4].
However, these protocols have resulted in a subset of patients exhibiting exceptionally favorable responses, including those classified as complete responders according to treatment criteria. Patients in this category who have undergone non-operative management have been reported to experience oncologic outcomes comparable to those who received radical resection [
5,
6]. This context has fostered the emergence of organ-preserving strategies, most notably the watch and wait (WW) approach, which has quickly gained prominence and has become a central focus in rectal cancer management [
5-
8]. Nonetheless, significant limitations persist in the broader application of these strategies, particularly regarding the lack of reliable criteria for assessing treatment response and standardized surveillance protocols.
The present review evaluates the treatment outcomes of non-operative management, discusses current efforts to improve patient survival, and delineates the existing constraints on real-world applications.
The paradigm shift: rationale and emergence of watch and wait
The shift toward the WW strategy is fundamentally motivated by the goal of minimizing the substantial morbidity associated with radical surgery for rectal cancer, while still achieving comparable oncologic outcomes. Although TME provides effective local tumor control, it frequently results in severe and persistent side effects, including permanent colostomy, impaired bowel function (urgency, clustering, fecal incontinence), and sexual or urinary dysfunction—all of which significantly compromise patients’ quality of life [
2,
3].
Evidence that a notable proportion of patients—approximately 10%–25%, rising to 30%–60% with the advent of total neoadjuvant therapy (TNT)—achieve a pathological complete response (pCR) has challenged the need for radical surgery in all cases [
2,
9,
10]. The pioneering work of Dr. Angelita Habr-Gama, who first introduced the WW concept in the early 2000s, was instrumental in this shift. Her group established strict criteria for identifying clinical complete response (cCR) using comprehensive assessments, including digital rectal examination (DRE), endoscopy, and imaging [
5]. Early experiences from this group demonstrated that sustained tumor-free intervals could be achieved without surgery and that salvage treatments following local regrowth were successful, providing key evidence supporting the WW approach [
5].
The development of TNT has further increased the feasibility of WW. TNT, which is defined as the administration of all chemotherapy and radiotherapy prior to surgical intervention, aims to achieve earlier systemic control of micrometastatic disease, improve patient adherence, reduce toxicity, and significantly increase tumor regression and pCR rates [
9-
13]. Landmark studies, including the RAPIDO and PRODIGE trials, have demonstrated significantly higher pCR rates with TNT compared to conventional nCRT, thereby expanding the pool of candidates eligible for WW [
12,
13]. As a result, the WW strategy has evolved from a mere avoidance of surgery to a deliberate deferral of surgery, contingent on rigorous surveillance and continuous monitoring of clinical response [
10].
Importantly, the WW approach is more accurately described as “deferral of surgery” rather than “no surgery.” Robust salvage pathways are essential, as highlighted by the high success rates (88%–95.4%) reported for salvage surgery in cases of local tumor regrowth [
10,
14]. The effectiveness and availability of salvage surgery reinforce the oncologic safety of the WW strategy. Therefore, ongoing, active surveillance is integral to WW, ensuring prompt detection and management of any tumor recurrence.
Ethics statement
This is a literature-based study; therefore, neither approval by an institutional review board nor informed consent is required.
Oncologic outcomes of watch and wait: a critical evaluation
The oncologic outcomes associated with the WW strategy for LARC have shown inconsistent results. While some reports are promising, demonstrating outcomes equivalent to those of standard radical surgical treatment, others highlight areas that require caution and further investigation.
Overall survival, disease-free survival, and disease-specific survival
Many studies and meta-analyses have reported comparable overall survival (OS) and disease-free survival (DFS) rates between patients managed with WW and those undergoing radical surgery after achieving cCR or pCR [
3]. A pooled analysis of the CAO/ARO/AIO-12 and OPRA trials, which included 628 patients, found similar survival outcomes between selective WW and mandatory TME in patients with cCR or near-complete response (nCR). Specifically, the 3-year DFS (76% for WW vs. 73% for TME), distant recurrence-free survival, local recurrence-free survival, and OS were all equivalent [
15]. Additionally, recent cohort analyses have reported no significant differences in OS, 5-year DFS, rates of distant metastasis, or mortality between WW and surgical groups [
16-
18].
However, some studies advise greater caution. One retrospective analysis comparing WW patients with cCR to pCR patients who underwent surgery reported lower survival rates for the WW group (5-year OS: 73% for WW vs. 94% for pCR; DFS: 75% for WW vs. 92% for pCR) [
19]. Notably, this study identified important confounding variables, such as a higher median age in the WW group (67.2 years vs. 57.3 years) and a substantial proportion (70%) of deaths attributable to non-cancer-related causes, both of which could influence overall survival comparisons [
19]. According to real-world data from the International Watch & Wait Database (IWWD), the 2-year cumulative incidence of local regrowth was 25.2%. Conditional survival analysis indicated that patients who maintained cCR for 3 years had less than a 2% risk of systemic recurrence thereafter, during a median long-term follow-up of 55.2 months [
20]. Consequently, the initial 2 years following completion of nCRT are crucial for prognostication and early detection of recurrence.
A major concern with WW is the risk of local tumor regrowth, with reported rates ranging from 6% to 40%, depending on study design and length of follow-up [
8,
10,
19]. For example, the IWWD documented a 2-year cumulative incidence of local regrowth at 25% [
8,
20]. The OPRA trial reported local regrowth rates ranging from 27% to 40%, depending on the specific neoadjuvant therapy regimen [
10].
The salvage rate for regrowth following the WW strategy varies across published studies [
8,
16,
19,
20]. Nonetheless, the majority of local regrowths can be detected early through rigorous surveillance and are amenable to effective salvage surgery. Outcomes of salvage surgery are generally favorable, with high rates of curative intent. Therefore, the feasibility and success of salvage surgery are crucial in reinforcing the oncologic safety of the WW approach.
Despite successful local control achieved through salvage surgery, concerns remain regarding the risk of distant metastases. Multiple studies have shown higher rates of distant metastasis in patients who experience local regrowth compared to those who maintain continuous cCR (36% vs. 1%, respectively; P<0.001) [
19]. Indeed, local regrowth following WW is now recognized as a significant independent risk factor for subsequent distant metastases [
14]. According to the IWWD, distant metastases occurred in approximately 8% of patients managed with WW [
20].
These findings suggest that an undetected primary tumor left in situ until regrowth may contribute to systemic disease progression, indicating that local regrowth could serve as an early marker of aggressive tumor biology or incomplete systemic response to treatment [
20].
While WW demonstrates favorable outcomes when proper patient selection and surveillance are applied, it is essential to acknowledge that these outcomes are highly dependent on both early detection and successful salvage surgery for local recurrence. As such, the oncologic safety of WW critically depends on 2 key factors: the prompt identification of local regrowth and the effectiveness of subsequent salvage interventions.
Additionally, the consistently higher rates of distant metastasis observed among WW patients experiencing local regrowth suggest a potential systemic risk associated with deferring surgery. This raises important questions about whether organ preservation might inadvertently compromise systemic disease control in certain patient subgroups. Currently, the use of TNT has expanded considerably, and its oncologic outcomes have been shown to be superior to standard nCRT (
Tables 1,
2), which is likely to further accelerate the adoption of WW. Future research must therefore focus on identifying and stratifying high-risk patients who may require intensified systemic therapy or who might not be ideal candidates for WW prior to any local recurrence.
Challenges in defining cCR
A major obstacle to the widespread adoption and standardization of the WW approach is the absence of a universally accepted and highly accurate definition of cCR [
10,
20,
21]. At present, cCR is defined by the absence of a clinically detectable tumor on DRE, endoscopy, and imaging [
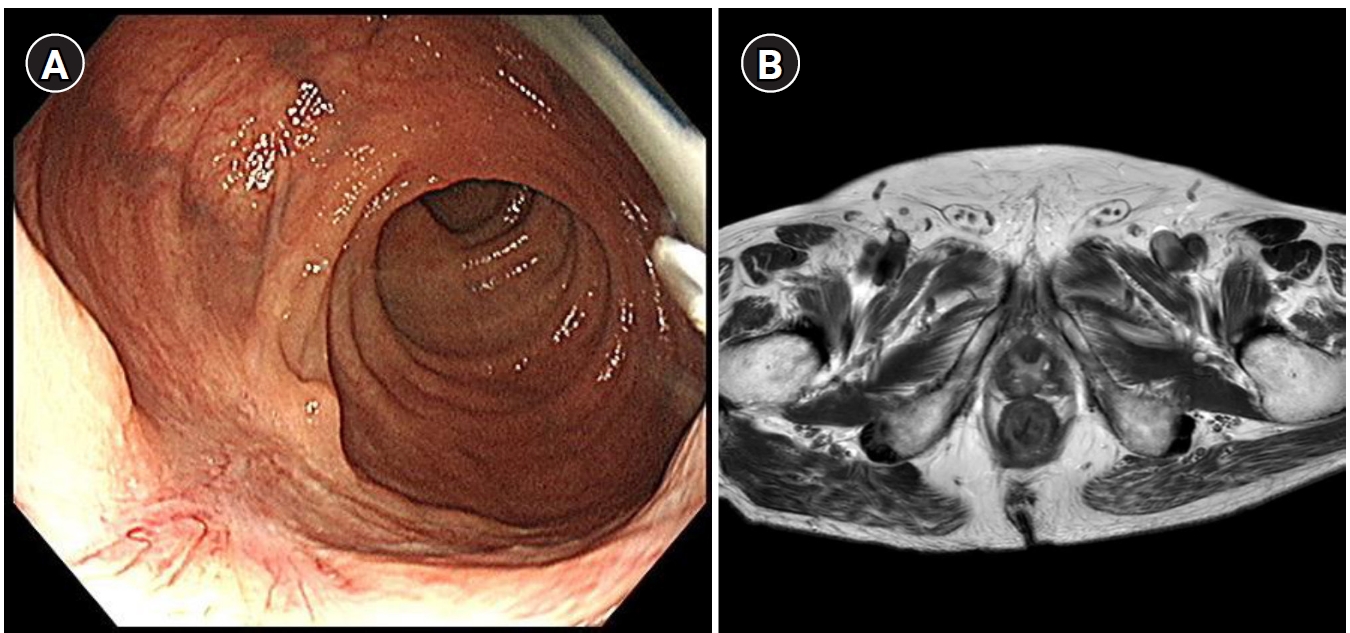
10] (
Fig. 1). However, these methods have significant limitations in reliably identifying true complete tumor regression, particularly at the microscopic level.
The primary challenge in determining cCR is differentiating between complete tumor regression and microscopic residual disease, as well as distinguishing post-treatment fibrosis or inflammation from viable cancer cells [
22,
23]. While pCR—the absence of viable tumor cells in resected surgical specimens—remains the definitive gold standard, pCR can only be confirmed after surgery and thus cannot guide the initial decision to pursue WW. Thus, discrepancies between cCR and actual pCR raise considerable concerns, as inaccurate clinical assessments may lead to understaging, increased risk of local regrowth, and potentially compromised oncologic outcomes.
Currently available diagnostic modalities, such as magnetic resonance imaging (MRI) and endoscopic biopsy, lack the sensitivity to detect microscopic residual disease, especially if tumor cells persist in deeper layers of the rectal wall [
23,
24]. Studies have shown that even patients categorized as having achieved cCR may harbor deeper residual tumor cells, directly contributing to local regrowth and increased risk of distant metastasis [
23-
26]. This diagnostic gap underscores the intrinsic limitations of existing non-invasive methods and highlights the urgent need for improved techniques capable of accurately detecting microscopic residual disease. Until such advances are achieved, strict surveillance and robust salvage strategies must remain central to WW protocols.
Careful patient selection is fundamental to optimizing oncologic outcomes in WW. At present, candidates generally include those with LARC who achieve an excellent clinical response to neoadjuvant therapy [
20,
21]. Recently, some reports have proposed simplified response criteria after nCRT for rectal cancer, introducing the concept of a transient response group characterized as slow responders or those with near-complete response [
27,
28]. However, caution is warranted in selecting patients from this group, as the oncologic safety of WW in slow responders has not been clearly established.
Patient preferences and the ability to adhere to stringent surveillance schedules also play a critical role in patient selection [
29-
31]. WW is particularly appropriate for patients with low rectal tumors, where surgery would cause substantial impairment of quality of life, or for older and frail patients with significant comorbidities who are at elevated perioperative risk [
31]. Thus, optimal patient selection demands a comprehensive, multidimensional assessment, balancing oncologic risks against anticipated quality-of-life gains and individual patient factors. There is a pressing need for better risk stratification tools to refine selection criteria and further personalize the WW approach. Effective implementation requires a coordinated multidisciplinary team and shared decision-making, ensuring that patients are fully informed of the potential risks and benefits.
Despite substantial progress, current diagnostic and surveillance methods for determining cCR have notable limitations that hinder the consistent application and widespread standardization of WW.
DRE and endoscopy remain the cornerstone modalities for cCR assessment, enabling direct palpation and visualization of the rectal tumor bed [
20,
27,
32]. However, these techniques are highly operator-dependent, reducing reproducibility and increasing the likelihood of missing subtle mucosal changes, submucosal residual disease, or deeper malignant foci. Endoscopic biopsy, in particular, is limited by its inability to adequately sample deeper layers, especially the muscularis propria, which leads to false-negative results [
27].
MRI (1.5 T or 3 T) is the imaging method of choice for both initial staging and post-treatment assessment, providing excellent soft-tissue differentiation and visualization of mesorectal structures and lymph nodes [
22,
33]. Nevertheless, MRI often struggles to distinguish viable tumor from post-treatment fibrosis, edema, or inflammation, resulting in reduced accuracy for restaging after neoadjuvant therapy. The accuracy of MRI for post-treatment T-staging may be as low as 50%, and nodal staging accuracy is generally between 60% and 80% [
34,
35]. Additionally, variability in MRI interpretation, stemming from non-standardized reporting practices across institutions, further contributes to inconsistencies [
23]. Therefore, imaging findings such as restricted diffusion or abnormal nodal morphology may suggest residual disease, but their interpretation is challenging due to significant overlap with benign post-treatment changes [
27].
The intrinsic challenge of distinguishing fibrosis and inflammatory tissue from viable residual cancer cells on imaging—especially MRI—remains a significant diagnostic hurdle. Fibrosis induced by chemoradiotherapy often closely mimics residual disease radiologically, leading to both false-negative and false-positive assessments. This diagnostic ambiguity contributes directly to the uncertainty associated with WW outcomes and underscores the need for rigorous surveillance protocols to mitigate the risk of local regrowth. Addressing these challenges requires the development of more precise, objective imaging criteria and advanced imaging technologies capable of accurately differentiating fibrosis from viable tumor cells.
Furthermore, the high operator dependency of clinical assessments (DRE, endoscopy, endorectal ultrasound [ERUS]) and the marked variability in MRI interpretation between institutions further compromise the reproducibility and generalizability of cCR determinations. This variability undermines confidence in outcomes reported from WW registries and highlights the urgent need for standardized assessment protocols, enhanced clinician and radiologist training, and the integration of artificial intelligence (AI) and other quantitative tools to reduce inter-observer variability and improve diagnostic accuracy [
36].
Establishing standardized surveillance protocols
Given the significant diagnostic challenges in defining cCR and the substantial risk of local tumor regrowth in patients managed with WW, the development of standardized and rigorously coordinated surveillance protocols is essential to ensuring patient safety and optimizing outcomes. The effectiveness of WW is fundamentally dependent on the timely detection and successful surgical management of local regrowth, underscoring the necessity for intensive and structured surveillance strategies [
20,
21,
37].
Early WW protocols, most notably those established by Habr-Gama, employ intensive monitoring regimens that include frequent DREs, carcinoembryonic antigen testing, endoscopic assessments, and MRI. A typical surveillance schedule involves monthly to bimonthly evaluations during the first year, quarterly evaluations in the second year, and semiannual assessments from the third year onward. Additionally, serial MRI scans are commonly performed every 6 months once an initial cCR is confirmed [
6,
20,
37]. Many institutions implement similar protocols, generally conducting assessments every 3 months during the first 2 years and every 6 months thereafter until year 5.
Although WW strategies are increasingly adopted worldwide, there remains considerable variability among institutions with respect to patient selection, treatment protocols, and surveillance regimens [
19,
20,
30,
38]. The National Accreditation Program for Rectal Cancer (NAPRC), for example, acknowledges the WW approach but does not provide specific clinical management or follow-up guidelines, leaving these decisions to the discretion of individual multidisciplinary teams. This lack of standardization is concerning, as inconsistent approaches can delay detection of tumor regrowth, potentially jeopardizing the outcomes of salvage surgery and increasing the risk of distant metastasis.
Given the relatively high local regrowth rates (15%–40%) [
17,
20,
21], paired with high salvage surgery success rates (90%–95%) [
37], the WW strategy should be recognized as an active management approach, not a passive observational one. Thus, the oncologic safety of WW relies less on the absolute prevention of recurrence and more on reliably detecting and effectively treating recurrence when it occurs. In this context, surveillance is not merely routine follow-up but a vital therapeutic component, transforming the paradigm from “watch and wait” to “watch, detect, and intervene promptly.”
However, there remains a gap between the ideal and the practical. While rigorous, multimodal surveillance protocols are critical for patient safety, their intensity, duration, and resource demands can be challenging for patients and healthcare systems to sustain and implement equitably [
39]. The resource-intensive nature of such protocols necessitates significant expertise and training among radiologists, endoscopists, and other specialists [
40]. As a result, translating the theoretical benefits of WW into standard clinical practice is often constrained by logistical barriers, patient compliance issues, and disparities in access to specialized care.
Addressing these challenges requires ongoing research aimed at optimizing surveillance strategies, striking a balance between protocol rigor, practical feasibility, patient acceptability, cost-effectiveness, and equitable access. The establishment of accredited WW centers of excellence will also be necessary to ensure quality assurance, standardized protocols, and fair patient access to specialized care pathways.
Role of multidisciplinary teams
The successful implementation of WW depends on the active participation of a highly coordinated multidisciplinary team (MDT), including surgeons, medical and radiation oncologists, radiologists, pathologists, and specialized endoscopists. The MDT must ensure accurate interpretation of clinical, radiological, and pathological findings to provide comprehensive patient assessment and ongoing monitoring. Effective shared decision-making, characterized by transparent communication regarding risks and benefits, is critical for promoting patient understanding and securing adherence to demanding surveillance protocols.
Future directions and ongoing research
The continued advancement and broader adoption of WW strategy in rectal cancer management depend significantly on dedicated research efforts. Key efforts include optimal patient selection, enhancing the accuracy of clinical response assessment, and developing effective surveillance protocols.
Current research seeks to enhance the detection of microscopic residual disease through advanced imaging technologies. Radiomics enables the extraction of detailed quantitative features from standard MRI scans, improving the prediction of cCR by correlating imaging findings with pathology and genomic data [
41]. Functional MRI techniques are being investigated to better differentiate residual viable tumor from fibrosis or treatment-induced changes [
42]. Novel modalities such as endorectal photoacoustic ultrasound are also under study to improve tumor response evaluation. Additionally, AI and machine learning methods are increasingly applied, utilizing deep learning algorithms to analyze imaging data and achieve more accurate assessments of treatment response [
36,
43].
There is an urgent need for non-invasive, highly sensitive, and specific biomarkers that can reliably predict which patients will achieve a cCR and are suitable for WW. Circulating tumor DNA, detected through liquid biopsies, has shown promise for identifying microscopic residual cancer cells and for predicting both response and recurrence [
44,
45]. However, current circulating biomarkers still lack sufficient specificity and clinical evidence, necessitating further validation and standardization before widespread clinical adoption.
Recent research also focuses on optimizing and intensifying TNT regimens to achieve even higher rates of complete response [
46]. The OPRA trial demonstrated that long-course chemoradiotherapy followed by consolidation chemotherapy produced superior outcomes in achieving cCR and increased TME-free survival, making it a preferred approach for organ preservation [
10,
15]. Additional studies are exploring whether the duration and intensity of TNT regimens can be safely reduced to minimize toxicity without compromising efficacy [
10-
14].
Many international observational studies and clinical trials continue to validate the safety, feasibility, and efficacy of nonoperative management [
16,
20,
47]. These studies are essential for gathering long-term outcome data and for resolving controversies regarding patient selection, cCR diagnosis, and optimal surveillance protocols. Patients undergoing WW should ideally be enrolled in prospective registries or clinical trials to contribute to this evolving evidence base. The integration of genetic profiles, molecular markers, and AI-driven predictive models represents a strong trend toward personalized medicine, enabling clinicians to tailor treatment strategies that maximize organ preservation while ensuring oncologic safety.
Conclusion
The WW strategy marks a significant evolution in the management of LARC, shifting the paradigm away from routine radical surgery and toward prioritizing organ preservation and quality of life. Its feasibility has increased with the higher rates of cCR achieved through TNT.
Despite promising survival outcomes comparable to those of standard radical surgery in patients who respond well to nCRT, important challenges persist. High rates of local regrowth remain a concern, even though salvage surgery is generally effective. Moreover, the elevated risk of distant metastasis among patients experiencing local recurrence underscores that WW is fundamentally a “deferral of surgery” rather than an outright avoidance.
Accurately identifying cCR remains a limitation, as current diagnostic tools struggle to distinguish true cCR from microscopic residual disease or post-treatment fibrosis. This complicates patient selection and mandates intensive surveillance. An alternative—careful selection of patients who may achieve a complete response during a transient observation period—has been suggested, but consensus and supporting evidence are still lacking. The lack of universally accepted and standardized surveillance protocols further restricts the widespread and equitable implementation of WW.
Future research aims to overcome these barriers through advancements in imaging technologies, AI-powered diagnostics, and the development of novel biomarkers. Ongoing clinical trials and patient registries are expected to yield essential long-term evidence to refine and optimize the WW approach.
Ultimately, the successful implementation of the WW strategy depends on a careful balance between organ preservation and oncologic safety. Rigorous patient selection, accurate diagnostic modalities, standardized surveillance protocols, and multidisciplinary shared decision-making are all essential to ensure that WW delivers optimal outcomes for patients.
-
Authors’ contributions
All work was done by In Ja Park
-
Conflict of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Data availability
Not applicable.
-
Acknowledgments
None.
-
Supplementary materials
None.
Fig. 1.Imaging diagnosis of clinical complete response after neoadjvuant therapy for rectal cancer. (A) Endoscpic image showing a whitish scar. (B) Absence of tumor signal and barely visible treatment-related sacr on magnetic resonance imaging.
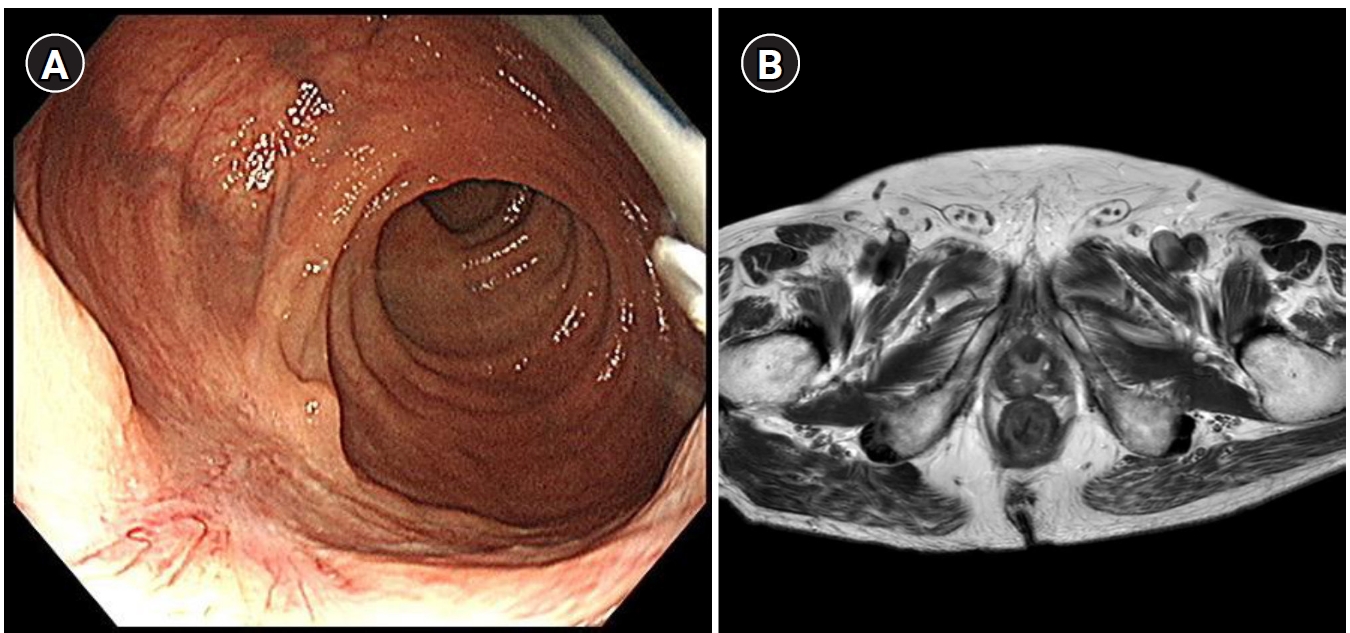
Table 1.Location of clinical stage of patients included in TNT trials for rectal cancer
|
Trial |
Location (cm from anal verge) |
Diagnosis method |
Detailed indication |
|
nCRT vs. TNT |
|
|
|
|
RAPIDO [13] |
<16 |
MRI |
High risk on pelvic MRI (with at least one of the following criteria: cT stage cT4a or cT4b, extramural vascular invasion, cN stage cN2, involved mesorectal fascia, or enlarged lateral lymph nodes) |
|
STELLAR [17] |
10 |
MRI |
cT stage 3–4 and/or regional lymph node (N)–positivity |
|
PRIODIGE 23 [14] |
<15 |
ERUS/MRI |
Stage cT3–4 (based ERUS/MRI) |
|
Induction vs. consolidation TNT |
|
|
|
|
CAO-ARO-AIO 12 [18] |
<12 |
MRI |
cT3 tumor less than 6 cm from the anal verge, cT3 tumor in the middle third of the rectum (≥6–12 cm) with extramural tumor spread into the mesorectal fat of more than 5 mm (>cT3b), cT4 tumors, or lymph node involvement, based on MRI that was mandatory. |
|
OPRA [10] |
Require complete TME |
MRI |
Clinical stage II (T3–4, N-) or stage III (any T, N+) based on MRI |
Table 2.Pathologic and oncologic outcomes of TNT trials for rectal cancer
|
Result |
R0 rate |
pCR |
APR rate |
Local recurrence |
Disease-free survival |
Overall survival |
|
nCRT vs. TNT |
|
|
|
|
|
|
|
RAPIDO [13] |
- |
|
|
|
|
|
|
TNT |
- |
28 |
35 |
12.1 |
27.8*
|
81.7 |
|
nCRT |
- |
14 |
40 |
8.1 |
34.6*
|
80.2 |
|
STELLAR [17] |
|
|
|
|
|
|
|
TNT |
91.5 |
21.8 |
45.1 |
8.4 |
64.5 |
86.5 |
|
nCRT |
87.8 |
12.3*
|
41.3 |
11.0 |
62.3 |
75.1*
|
|
PRIODIGE 23 [14] |
|
|
|
|
|
|
|
TNT |
95 |
28 |
14.1 |
|
67.6 |
81.9 |
|
nCRT |
94 |
12 |
14 |
|
62.5*
|
76.1*
|
|
Induction vs. consolication TNT |
|
|
|
|
|
|
|
CAO-ARO-AIO 12 [18] |
|
|
|
|
|
- |
|
Consolidation |
92 |
17 |
28 |
6 |
73 |
- |
|
Induction |
90 |
25*
|
23 |
5 |
73 |
- |
|
OPRA [10] |
- |
- |
- |
- |
|
- |
|
Consolidation |
- |
- |
- |
- |
71 |
- |
|
Induction |
- |
- |
- |
- |
69 |
- |
References
- 1. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rodel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-1933. https://doi.org/10.1200/JCO.2011.40.1836
- 2. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-582. https://doi.org/10.1016/S1470-2045(11)70097-3
- 3. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-1740. https://doi.org/10.1056/NEJMoa040694
- 4. Chong CX, Koh FH, Tan HL, Sivarajah SS, Ng JL, Ho LM, Aw DK, Koo WH, Han S, Koo SL, Yip CS, Wang FQ, Foo FJ, Tan WJ. The impact of short-course total neoadjuvant therapy, long-course chemoradiotherapy, and upfront surgery on the technical difficulty of total mesorectal excision: an observational study with an intraoperative perspective. Ann Coloproctol 2024;40:451-458. https://doi.org/10.3393/ac.2023.00899.0128
- 5. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-718. https://doi.org/10.1097/01.sla.0000141194.27992.32
- 6. Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewe KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633-4640. https://doi.org/10.1200/JCO.2011.37.7176
- 7. Dulskas A, Caushaj PF, Grigoravicius D, Zheng L, Fortunato R, Nunoo-Mensah JW, Samalavicius NE. International Society of University Colon and Rectal Surgeons survey of surgeons’ preference on rectal cancer treatment. Ann Coloproctol 2023;39:307-314. https://doi.org/10.3393/ac.2022.00255.0036
- 8. van der Valk MJ, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJ. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018;391:2537-2545. https://doi.org/10.1016/S0140-6736(18)31078-X
- 9. Patel S, Ankathi S, Haria P, Kazi M, Desouza AL, Saklani A. Impact of consolidation chemotherapy in poor responders to neoadjuvant radiation therapy: magnetic resonance imaging-based clinical-radiological correlation in high-risk rectal cancers. Ann Coloproctol 2023;39:474-483. https://doi.org/10.3393/ac.2023.00080.0011
- 10. Verheij FS, Omer DM, Williams H, Lin ST, Qin LX, Buckley JT, Thompson HM, Yuval JB, Kim JK, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Guillem JG, Temple L, Goodman KA, Segal NH, Cercek A, Yaeger R, Nash GM, Widmar M, Wei IH, Pappou EP, Weiser MR, Paty PB, Smith JJ, Wu AJ, Gollub MJ, Saltz LB, Garcia-Aguilar J. Long-term results of organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy: the randomized phase II OPRA trial. J Clin Oncol 2024;42:500-506. https://doi.org/10.1200/JCO.23.01208
- 11. Sychev S, Ponomarenko A, Chernyshov S, Alekseev M, Mamedli Z, Kuzmichev D, Polynovskiy A, Rybakov E. Total neoadjuvant therapy in rectal cancer: a network meta-analysis of randomized trials. Ann Coloproctol 2023;39:289-300. https://doi.org/10.3393/ac.2022.00920.0131
- 12. Conroy T, Bosset JF, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouche O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardiere C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702-715. https://doi.org/10.1016/S1470-2045(21)00079-6
- 13. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CA, Putter H, Kranenbarg EM, Roodvoets AG, Nagtegaal ID, Beets-Tan RG, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJ, Hospers GA. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. https://doi.org/10.1016/S1470-2045(20)30555-6
- 14. Lin W, Wee IJ, Seow-En I, Chok AY, Tan EK. Survival outcomes of salvage surgery in the watch-and-wait approach for rectal cancer with clinical complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Ann Coloproctol 2023;39:447-456. https://doi.org/10.3393/ac.2022.01221.0174
- 15. Williams H, Fokas E, Diefenhardt M, Lee C, Verheij FS, Omer DM, Lin ST, Dunne RF, Marcet J, Cataldo P, Polite B, Piso P, Polat B, Dapper H, Ghadimi M, Hofheinz RD, Qin LX, Saltz LB, Wu AJ, Gollub MJ, Smith JJ, Weiser MR, Rodel C, Garcia-Aguilar J. Survival among patients treated with total mesorectal excision or selective watch-and-wait after total neoadjuvant therapy: a pooled analysis of the CAO/ARO/AIO-12 and OPRA randomized phase II trials. Ann Oncol 2025;36:543-547. https://doi.org/10.1016/j.annonc.2025.01.006
- 16. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O’Dwyer ST. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174-183. https://doi.org/10.1016/S1470-2045(15)00467-2
- 17. Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, Liu WY, Chen SL, Li S, Lu NN, Cai Y, Li YH, Zhu Y, Cheng GH, Zhang HY, Wang X, Zhu SY, Wang J, Li GF, Yang JL, Zhang K, Chi Y, Yang L, Zhou HT, Zhou AP, Zou SM, Fang H, Wang SL, Zhang HZ, Wang XS, Wei LC, Wang WL, Liu SX, Gao YH, Li YX. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol 2022;40:1681-1692. https://doi.org/10.1200/JCO.21.01667
- 18. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Kirste S, Jacobasch L, Allgauer M, Flentje M, Germer CT, Grutzmann R, Hildebrandt G, Schwarzbach M, Bechstein WO, Sulberg H, Friede T, Gaedcke J, Ghadimi M, Hofheinz RD, Rodel C. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol 2022;8:e215445. https://doi.org/10.1001/jamaoncol.2021.5445
- 19. Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LK, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol 2019;5:e185896. https://doi.org/10.1001/jamaoncol.2018.5896
- 20. Fernandez LM, Sao Juliao GP, Figueiredo NL, Beets GL, van der Valk MJ, Bahadoer RR, Hilling DE, Meershoek-Klein Kranenbarg E, Roodvoets AG, Renehan AG, van de Velde CJ, Habr-Gama A, Perez RO. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol 2021;22:43-50. https://doi.org/10.1016/S1470-2045(20)30557-X
- 21. Gani C, Fokas E, Polat B, Ott OJ, Diefenhardt M, Konigsrainer A, Boke S, Kirschniak A, Bachmann R, Wichmann D, Bitzer M, Clasen S, Grosse U, Hoffmann R, Gotz M, Hofheinz RD, Germer E, Germer CT, Fietkau R, Martus P, Zips D, Rodel C. Organ preservation after total neoadjuvant therapy for locally advanced rectal cancer (CAO/ARO/AIO-16): an open-label, multicentre, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol 2025;10:562-572. https://doi.org/10.1016/S2468-1253(25)00049-4
- 22. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-3760. https://doi.org/10.1200/JCO.2011.34.9068
- 23. Park SH, Cho SH, Choi SH, Jang JK, Kim MJ, Kim SH, Lim JS, Moon SK, Park JH, Seo N. MRI Assessment of complete response to preoperative chemoradiation therapy for rectal cancer: 2020 guide for practice from the Korean Society of Abdominal Radiology. Korean J Radiol 2020;21:812-828. https://doi.org/10.3348/kjr.2020.0483
- 24. van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive value of endoscopic features for a complete response after chemoradiotherapy for rectal cancer. Ann Surg 2021;274:e541-e547. https://doi.org/10.1097/SLA.0000000000003718
- 25. Williams H, Lee C, Garcia-Aguilar J. Nonoperative management of rectal cancer. Front Oncol 2024;14:1477510. https://doi.org/10.3389/fonc.2024.1477510
- 26. Wang QX, Zhang R, Xiao WW, Zhang S, Wei MB, Li YH, Chang H, Xie WH, Li LR, Ding PR, Chen G, Zeng ZF, Wang WH, Wan XB, Gao YH. The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol 2021;16:16. https://doi.org/10.1186/s13014-021-01746-0
- 27. Custers PA, Beets GL, Bach SP, Blomqvist LK, Figueiredo N, Gollub MJ, Martling A, Melenhorst J, Ortega CD, Perez RO, Smith JJ, Lambregts DM, Beets-Tan RG, Maas M. An international expert-based consensus on the definition of a clinical near-complete response after neoadjuvant (chemo)radiotherapy for rectal cancer. Dis Colon Rectum 2024;67:782-795. https://doi.org/10.1097/DCR.0000000000003209
- 28. Gambacorta MA, Masciocchi C, Chiloiro G, Meldolesi E, Macchia G, van Soest J, Peters F, Collette L, Gerard JP, Ngan S, Rodel CC, Damiani A, Dekker A, Valentini V. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol 2021;154:154-160. https://doi.org/10.1016/j.radonc.2020.09.026
- 29. Son GM. Organ preservation for early rectal cancer using preoperative chemoradiotherapy. Ann Coloproctol 2023;39:191-192. https://doi.org/10.3393/ac.2023.00409.0058
- 30. Habr-Gama A, Lynn PB, Jorge JM, Sao Juliao GP, Proscurshim I, Gama-Rodrigues J, Fernandez LM, Perez RO. Impact of organ-preserving strategies on anorectal function in patients with distal rectal cancer following neoadjuvant chemoradiation. Dis Colon Rectum 2016;59:264-269. https://doi.org/10.1097/DCR.0000000000000543
- 31. Jung WB. Beyond survival: a comprehensive review of quality of life in rectal cancer patients. Ann Coloproctol 2024;40:527-537. https://doi.org/10.3393/ac.2024.00745.0106
- 32. Thompson HM, Omer DM, Lin S, Kim JK, Yuval JB, Verheij FS, Qin LX, Gollub MJ, Wu AJ, Lee M, Patil S, Hezel AF, Marcet JE, Cataldo PA, Polite BN, Herzig DO, Liska D, Oommen S, Friel CM, Ternent CA, Coveler AL, Hunt SR, Garcia-Aguilar J. Organ preservation and survival by clinical response grade in patients with rectal cancer treated with total neoadjuvant therapy: a secondary analysis of the OPRA randomized clinical trial. JAMA Netw Open 2024;7:e2350903. https://doi.org/10.1001/jamanetworkopen.2023.50903
- 33. Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465-1475. https://doi.org/10.1007/s00330-017-5026-2
- 34. Celik H, Barlik F, Sokmen S, Terzi C, Canda AE, Sagol O, Sarioglu S, Unlu M, Bilkay Gorken I, Arican Alicikus Z, Oztop I. Diagnostic performance of magnetic resonance imaging in preoperative local staging of rectal cancer after neoadjuvant chemoradiotherapy. Diagn Interv Radiol 2023;29:219-227. https://doi.org/10.4274/dir.2022.221333
- 35. Liu B, Sun C, Zhao X, Liu L, Liu S, Ma H. The value of multimodality MR in T staging evaluation after neoadjuvant therapy for rectal cancer. Technol Health Care 2024;32:615-627. https://doi.org/10.3233/THC-220798
- 36. Kim M, Park T, Oh BY, Kim MJ, Cho BJ, Son IT. Performance reporting design in artificial intelligence studies using image-based TNM staging and prognostic parameters in rectal cancer: a systematic review. Ann Coloproctol 2024;40:13-26. https://doi.org/10.3393/ac.2023.00892.0127
- 37. Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822-828. https://doi.org/10.1016/j.ijrobp.2013.12.012
- 38. Meng C, Shu W, Sun L, Wu S, Wei P, Gao J, Shi J, Li Y, Yang Z, Yao H, Zhang Z. Rectal cancer approach strategies after neoadjuvant treatment: a systematic review and network meta-analysis. Int J Surg 2025;111:3078-3092. https://doi.org/10.1097/JS9.0000000000002290
- 39. Crean R, Glyn T, McCombie A, Frizelle F. Comparing outcomes and cost in surgery versus watch & wait surveillance of patients with rectal cancer post neoadjuvant long course chemoradiotherapy. ANZ J Surg 2024;94:1151-1160. https://doi.org/10.1111/ans.18916
- 40. Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, Kerin M. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol 2019;25:4850-4869. https://doi.org/10.3748/wjg.v25.i33.4850
- 41. Rai J, Mai DV, Drami I, Pring ET, Gould LE, Lung PF, Glover T, Shur JD, Whitcher B, Athanasiou T, Jenkins JT. MRI radiomics prediction modelling for pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review and meta-analysis. Abdom Radiol (NY) 2025;Apr 28 [Epub]. https://doi.org/10.1007/s00261-025-04953-5
- 42. Pham TT, Liney GP, Wong K, Barton MB. Functional MRI for quantitative treatment response prediction in locally advanced rectal cancer. Br J Radiol 2017;90:20151078. https://doi.org/10.1259/bjr.20151078
- 43. Boldrini L, Charles-Davies D, Romano A, Mancino M, Nacci I, Tran HE, Bono F, Boccia E, Gambacorta MA, Chiloiro G. Response prediction for neoadjuvant treatment in locally advanced rectal cancer patients-improvement in decision-making: a systematic review. Eur J Surg Oncol 2025;51:109463. https://doi.org/10.1016/j.ejso.2024.109463
- 44. Morais M, Pinto DM, Machado JC, Carneiro S. CtDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol 2022;48:218-227. https://doi.org/10.1016/j.ejso.2021.08.034
- 45. Nassar A, Aly NE, Jin Z, Aly EH. CtDNA as a predictor of outcome after curative resection for locally advanced rectal cancer: systematic review and meta-analysis. Colorectal Dis 2024;26:1346-1358. https://doi.org/10.1111/codi.17039
- 46. Liu J, Xu X, Zhong H, Yu M, Abuduaini N, Fingerhut A, Cai Z, Feng B. Optimizing total neoadjuvant therapy in locally advanced rectal cancer: risk stratification should not be overlooked. Future Oncol 2025;21:1951-1960. https://doi.org/10.1080/14796694.2025.2507560
- 47. Wang L, Zhang XY, Zhao YM, Li SJ, Li ZW, Sun YS, Wang WH, Wu AW. Intentional watch and wait or organ preservation surgery following neoadjuvant chemoradiotherapy plus consolidation CAPEOX for MRI-defined low-risk rectal cancer: findings from a prospective phase 2 Trial (PKUCH-R01 trial, NCT02860234). Ann Surg 2023;277:647-654. https://doi.org/10.1097/SLA.0000000000005507