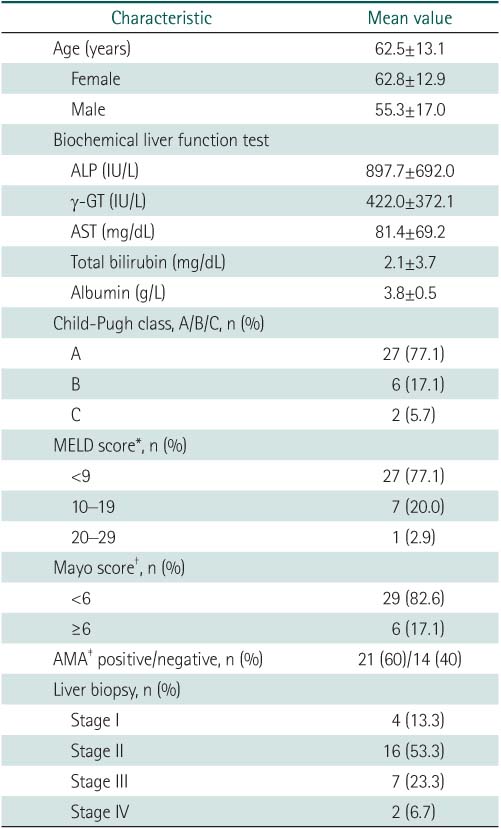
Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
1Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
Copyright © 2015, The Ewha Medical Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
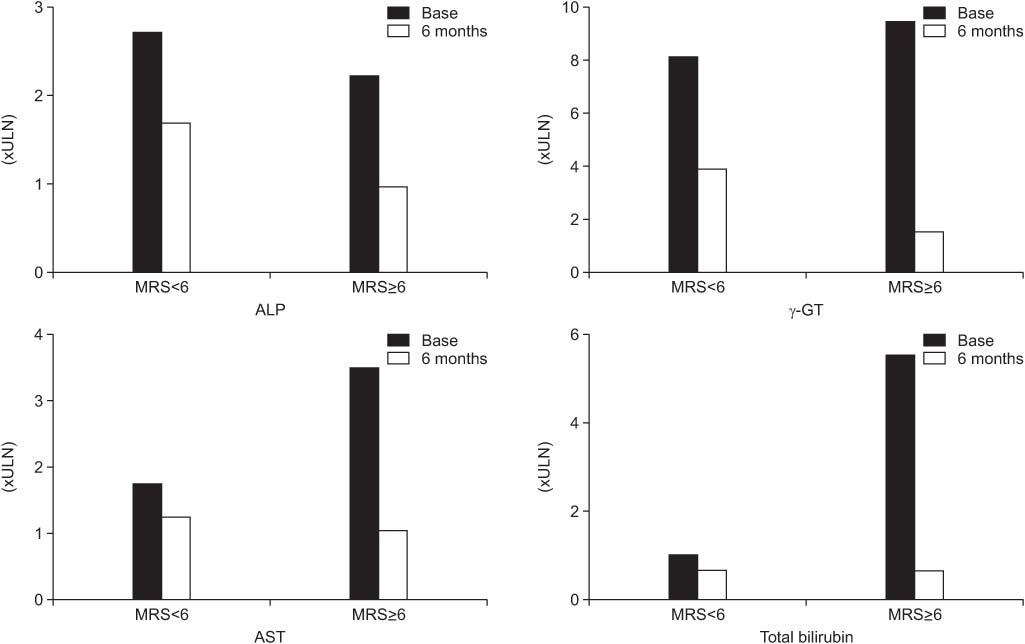
*MELD score [7], 2001: R=3.8xloge (bilirubin [mg/dL])+11.2xloge (INR) +9.6xloge (creatinine [mg/dL])+6.4x(etiology: 0 if cholestatic or alcoholic, 1 otherwise).
†Mayo score [6], 2000: age (0 point for 38, 1 for 38 to 62 and 2 for 63 years), bilirubin (0 point for 1, 1 for 1 to 1.7, 2 for 1.7 to 6.4, and 3 for 6.4 mg/dL), albumin (0 point for 4.1, 1 for 2.8 to 4.1, and 2 for 2.8 g/dL), prothrombin time (1 point for normal and 2 for prolonged), and edema (0 point for absent and 1 for present).
‡AMA, antimitochondrial antibody.

*MELD score [7], 2001: R=3.8xloge (bilirubin [mg/dL])+11.2xloge (INR) +9.6xloge (creatinine [mg/dL])+6.4x(etiology: 0 if cholestatic or alcoholic, 1 otherwise).
†Mayo score [6], 2000: age (0 point for 38, 1 for 38 to 62 and 2 for 63 years), bilirubin (0 point for 1, 1 for 1 to 1.7, 2 for 1.7 to 6.4, and 3 for 6.4 mg/dL), albumin (0 point for 4.1, 1 for 2.8 to 4.1, and 2 for 2.8 g/dL), prothrombin time (1 point for normal and 2 for prolonged), and edema (0 point for absent and 1 for present).
‡AMA, antimitochondrial antibody.
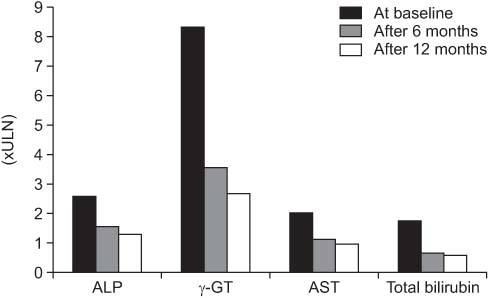
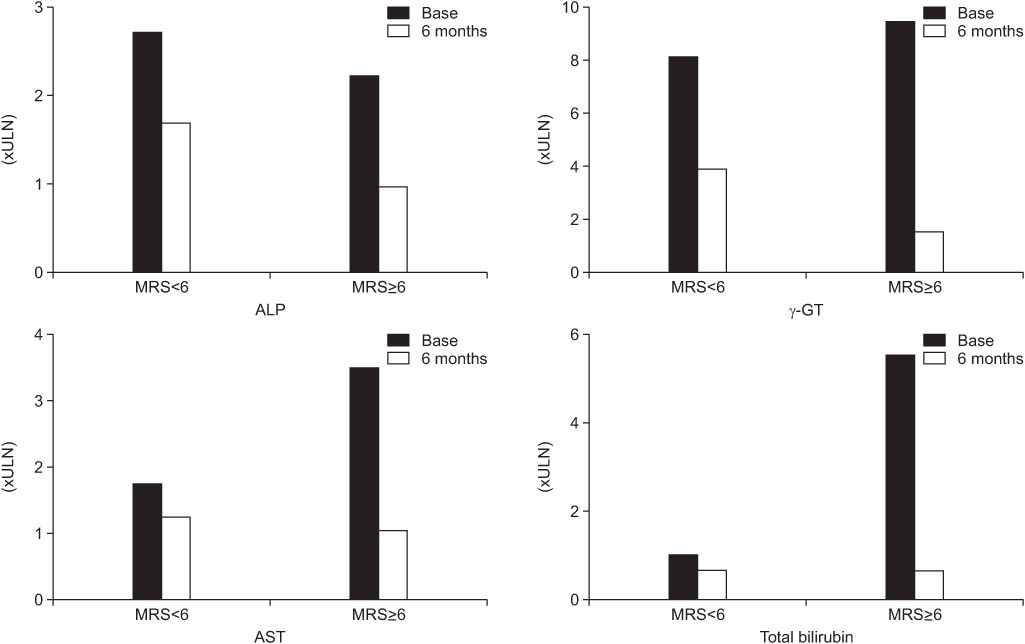
Values are presented as mean±SD.
*P<0.05.
ULN, upper limit of normal.
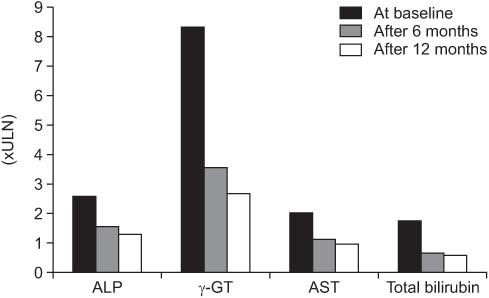
*MELD score [ †Mayo score [ ‡AMA, antimitochondrial antibody.
Values are presented as mean±SD. *P<0.05.
ULN, upper limit of normal.