Introduction
To determine the risk associated with anesthesia and surgery, anesthesiologists have focused on the issues of mortality and major morbidity such as myocardiac infarction, pneumonia and renal failure. Recently several studies have suggested that anesthesia itself can affect the endocrine and immunologic system, which has been underestimated traditionally. The alteration of endocrine and/or immunologic system occurs during perioperative period and affects the prognosis of the patients’ chronic underlying disease including malignant conditions [1-3]. Also, the excessive protein catabolism following major surgery is a main cause of morbidity and can lead to delay in recovery.
The insulin-like growth factors (IGFs) circulate in high concentrations bound to insulin-like growth factor binding proteins (IGFBPs). The IGFs, as a major regulating factor of cell differentiation, play a role to improve nitrogen retention, induce a wound recovery and is closely associated with a variety of conditions [4-7]. The IGFBPs transport IGFs and regulate the bioavailability and bioactivity of IGF, although the role of IGFBPs has not been fully understood. IGFBPs may either inhibit or potentiate IGF-mediated cell growth and metabolism depending on its concentration, cellular environment and post-translation modifications. To date, six different IGFBPs have been recognized. Because more than 95% of plasma IGFs are bound by IGFBP-3 together with the acid-labile subunit, many studies have focused on mainly IGFBP-3 [8,9].
Previous studies [10,11] suggested that the surgery could affect IGF and IGFBP profiles, however, no study has assessed the effect of the anesthetics used during surgery on the IGFBP. Therefore, in this study, we aimed to evaluate whether the IGFBP-3 level undergo major changes during perioperative periods according to the different anesthetic agents (propofol or isoflurane).
Methods
Eighteen adult American Society of Anesthesiologists physical status I–II patients scheduled for elective total abdominal hysterectomy due to uterine myoma were enrolled. The patients who had a history of renal, hepatic, endocrine, metabolic disturbances, malnutrition, receiving hormone medication, known allergies to any anesthetic drugs and pregnancy women were excluded.
Patients were assigned randomly to have either propofol (n=9) or isoflurane (n=9) for maintenance of general anesthesia using a computer-generated randomization table. All patients were fasted for 8 hours before surgery and were premedicated with midazolam 0.05 mg/kg, glycopyrrolate 0.2 mg 1 hour before surgery. On arrival in operation room, standard American Society of Anesthesiologists monitors were used throughout the surgery. In the propofol group, general anesthesia was induced with intravenous propofol (Fresofol MCT 2%, 20 mg/mL; Fresenius KABI, Graz, Austria), which was also used for intraoperative maintenance as a continuous infusion with a bolus of fentanyl 1 to 2 μg/kg. In the Isoflurane group, anesthesia was induced with 250 mg of thiopental sodium, fentanyl 1 to 2 μg/kg and maintained with inhalation of isoflurane. Tracheal intubation was facilitated with 0.5 mg/kg of vecuronium, which was also administered as subsequent incremental doses according to the requirements of the surgical procedure in the both groups. After tracheal intubation, ventilation was controlled to maintain end-tidal concentration of CO2 kept between 35 and 45 mmHg by adjusting the tidal volume with 50% nitrous oxide in oxygen using a fresh gas flow of 3 L/min. During an operation, the propofol and isoflurane were titrated to achieve bispectral index value between 40 and 60. Patients were extubated in the operating room after reversal of the residual muscle relaxation and transferred to the recovery room.
A 5 mL venous sample was taken for analysis of IGFBP-3 at the following time points: before induction, at the time of peritoneal closure, 1 hour after extubation at recovery room, and 2 and 5 postoperative days. The samples were collected and immediately centrifuged at 400 g for 15 minutes, and the serum was stored at -70oC until analyzed. The IGFBP-3 was determined by commercial ELISA (enzyme-linked immunosorbent assay) kit supplied by Diagnostic Systems Laboratories (Webster, TX, USA). Its detection limit was 0.04 ng/mL. Intra- and inter-assay coefficients of variation were 9.6% and 11.4%, respectively.
PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Kolmogorov-Smirnov was used to test a normal distribution of data. Discrete variables were analyzed with Fisher’s exact test. The Student’s t-test was used for comparing the IGFBP-3 concentration between groups. The repeated measures of analysis of variance was used for analyzing the IGFBP-3 concentration within each group. All results are presented as mean±standard deviation. Statistical significance was considered when the P-value was less than 0.05.
Results
Demographic and perioperative data including age, weight, body mass index, operation and anesthesia time and baseline blood sugar level were similar between groups (Table 1).
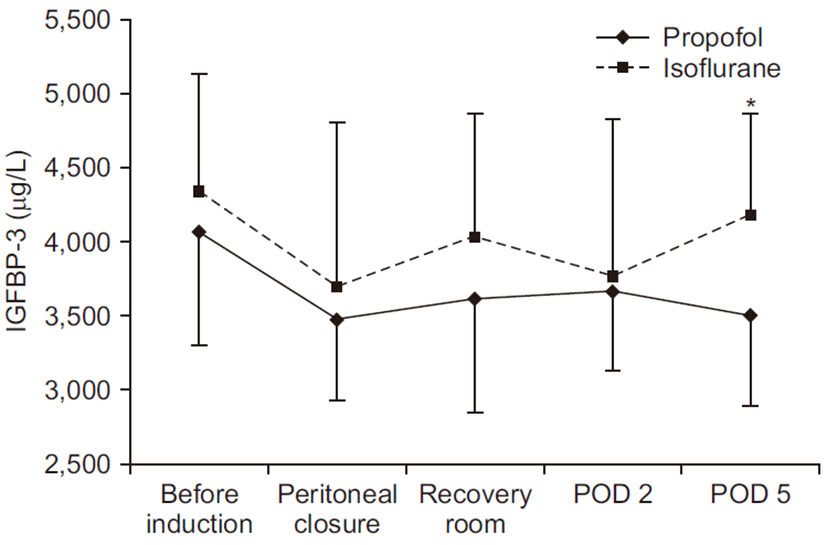
The levels of IGFBP-3 before induction were 4,070.00±773.48 μg/L in the propofol group and 4,341.22±791.70 μg/L in the isoflurane group, which were similar between groups. The average concentration of IGFBP-3 in all patients was 4,205.61±771.99 μg/L (range, 2,619 to 5,331 μg/L). In analysis within the propofol group, the IGFBP-3 concentration decreased throughout the study period, however, there was no statistically significance. In the isoflurane group, the IGFBP-3 concentration decreased after anesthesia induction, which also has no statistically significance. In both groups, the IGFBP-3 reached a nadir at the time of peritoneal closure. On the postoperative 5th day, the decreased concentration of IGFBP-3 in the isoflurane group was returned to preoperative value, whereas that in the propofol group was not recovered. There was a significant difference of the IGFBP-3 concentration between groups on the postoperative 5th day (P<0.05) (Fig. 1).
Discussion
A decreasing tendency of IGFBP-3 after anesthesia induction was observed without a statistically significant difference. Our study suggests that a different kind of general anesthesia agents can induce different effects on the IGFBP-3 level judging from the results that the decreased level of IGFBP-3 in the isoflurane group was recovered to preoperative value, whereas that observed in the propofol group was not recovered on the postoperative 5th day.
It has known that the IGFBP-3 level is not affected by the short-term fasting, circadian patterns or menstrual cycles [12-14]. It shows a steady age-dependent increase, peaking in the puberty, then declining slowly throughout adult life [15]. The different range of age of the both groups is considered to affect minimal effects on our results because the demographic data was similar in both groups.
In the previous study comparing the effects of surgery with those of fasting alone without surgery on the IGFBP-3 profiles, Davenport et al. [10] reported that the level of IGFBP-3 decreased markedly 2 days postoperatively and surgical factors other than nutritional factors were the principal regulators of the postoperative changes of IGFBP-3. The present study was designed to conduct in patients undergoing hysterectomy due to uterine myoma. Noh and Kim [16] described that the level of IGFBP-3 during perioperative periods did not differ between the healthy women and myoma patients. In that sense, it is thought that our results can be extended to that in the healthy women populations. The results by Noh and Kim [16] showed that the concentration of IGFBP-3 decreased 2 days postoperatively and returned to preoperative value on 6 days postoperatively, which is consistent with our results in the isoflurane group and previous data [10]. The study conducting in aged 30 to 70 years old patients, undergoing colorectal surgery by Cotterill et al. [11] showed that the IGFBP-3 level fell from the anesthesia induction and recovered to preoperative valued at 6 weeks. The discrepancy in the time of recovery to preoperative values may be attributable to surgical invasiveness, inclusion of old age populations and the anesthetics used for their study. The enflurane was used for anesthesia in their study by Cotterill et al. [11] and the others [10,16] did not indicate the anesthetics used for their study. It has been suggested that a decrease of the IGFBP-3 might make IGF more available for tissue uptake and help to counteract the catabolic state induced during perioperative periods by enhancing tissue uptake of IGF-1. It is probably related to specific circulating protease activity directed against IGFBP-3 [17,18]. The decrease in the IGFBP-3 is also observed in critically ill patients [19]. Although we measured the IGFBP-3 level only until 5 days postoperatively, it could be speculated that the use of propofol during operation made the patients adapt better to the catabolic state than the use of isoflurane. There is also a possibility that isoflurane might affect the proteolysis of IGFBP-3, which is observed in postoperative periods.
There are several limitations in our study. First, we designed the study in the relatively healthy patient population who does not have systemic disease besides uterine myoma. The pattern of IGFBP during severe illness is known to have major alterations when compared with that seen in the healthy patients [19]. The effect on endocrine system, which is associated with postoperative recovery and the progress of disease may be more important in critically ill patients than the healthy people. Second, we evaluated the effect on only IGFBP-3 and did not assess a correlation with the IGF. The IGFBP-3 is the major factor determining circulating IGF levels and closely related to IGF. An IGF and IGFBP-3 complex was found to accelerate wound healing better than IGF alone [20]. Third, it has been suggested that enzymatic proteolysis of IGFBP-3 by an IGFBP-3 specific protease after surgery might facilitate release of IGF and play a role to meet the extra demands. Additional evaluation about specific protease activity for IGFBP-3 may be valuable to understand the regulatory mechanism in the bioavailability of circulating IGF [17]. Fourthly, this study included a relatively small number of patients. Further study is needed to expand our results and provide a comprehensive interpretation.
In conclusion, this is the first study to show that the anesthetics used for general anesthesia can affect the IGFBP-3 level during perioperative periods. We have observed different patterns of perioperative IGFBP change when general anesthesia was maintained with propofol or isoflurane. Our results provide a basis for a randomized controlled trial in future investigations.