Introduction
Although blood pressure rises with age, it has been recognized that some individuals develop rapidly progressive blood pressure elevations with target organ injury. If these blood pressure elevations are not treated, the mortality rate in patients with target organ injury, including papilledema and declining kidney function, can exceed 50% over 6–12 months, and this hypertension is considered ‘malignant’ [1]. Malignant hypertension is histologically characterized by fibrinoid necrosis of arterioles and progresses rapidly. It is observed in a variety of organs, including the kidneys, pancreas, gastrointestinal tract, liver, retina, brain, myocardium, prostate, and skeletal muscles. However, pulmonary hemorrhage is rare. Here, we report a rare case of malignant hypertension with pulmonary alveolar hemorrhage.
Case
A 35-year-old man presented with cough, sudden onset progressive dyspnea, and hemoptysis. He had been previously healthy, with no history of any underlying disease.
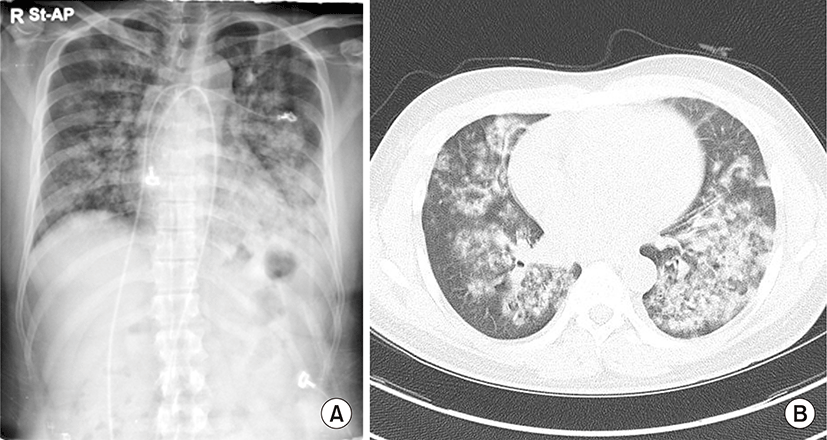
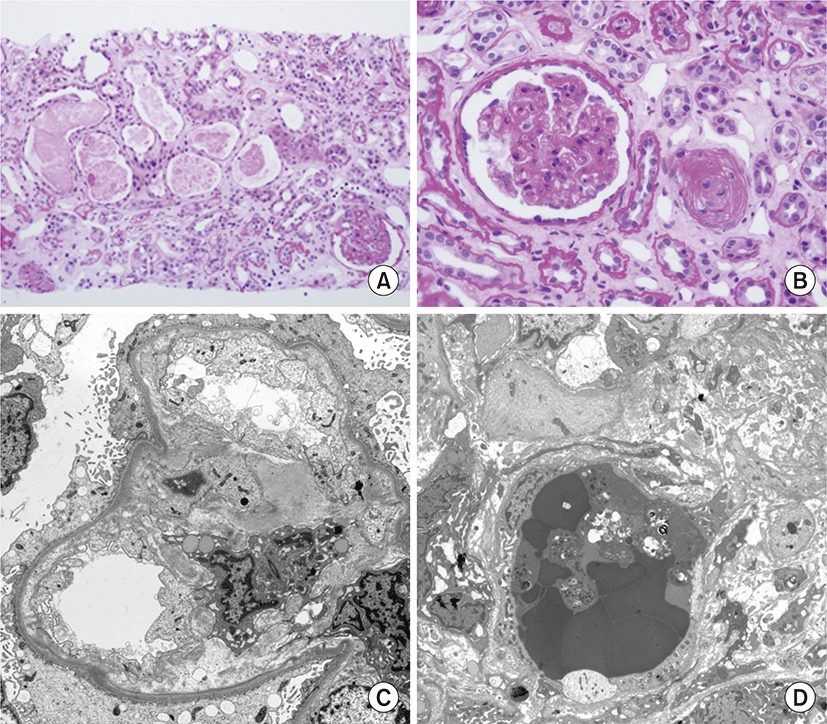
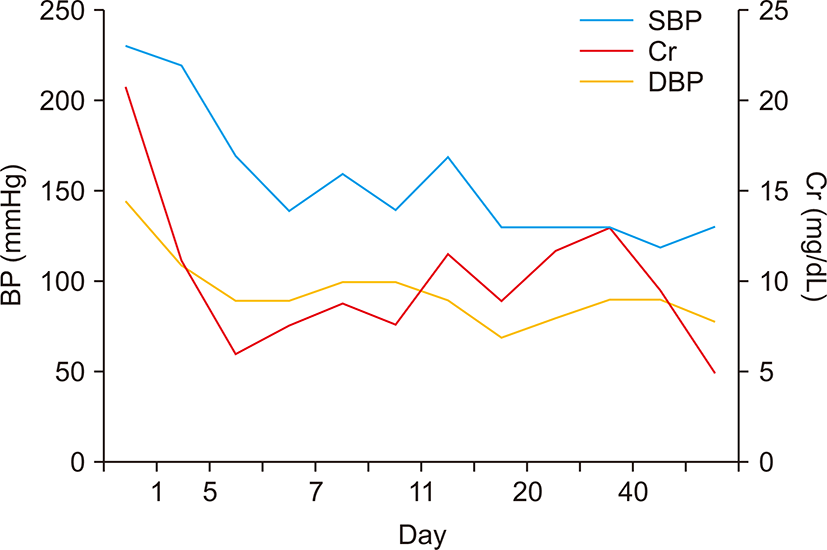
His blood pressure was 230/140 mmHg, and he had tachypnea (28 breaths/min), without fever. He did not have bilateral pretibial edema, and neurological examination did not reveal focal signs. Urinalysis showed 3+ proteinuria and hematuria. Laboratory examination revealed a hemoglobin level of 6.6 g/dL, hematocrit value of 18.2%, white blood cell count of 16,180/μL, platelet count of 89,000/μL, blood urea nitrogen level of 155 mg/dL, serum creatinine level of 20.13 mg/dL, and an estimated glomerular filtration rate of 2.84 mL/min/1.73 m2. Serum electrolyte analysis revealed a sodium level of 129 mEq/L, potassium level of 4.2 mEq/L, and chloride level of 90 mEq/L, with metabolic acidosis and respiratory alkalosis (pH, 7.42; pCO2, 22 mmHg; HCO3-, 14.3 mEq/L). A peripheral blood smear indicated normocytic normochromic anemia with schistocytes, anisopoikilocytosis, and polychromasia, and thrombocytopenia compatible with microangiopathic hemolytic anemia such as thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, or reactive conditions such as infection and inflammation. Fundoscopic examination showed retinal vein dilation, retinal artery thinning, cotton wool spots, papillary exudate, and macular pucker consistent with hypertension. Plasma renin activity, and aldosterone, vanillylmandelic acid, metanephrine, epinephrine, and norepinephrine levels were within normal limits. Autoantibodies, including anti-glomerular basement membrane, anti-nuclear, anti-ds-DNA, anti-ss-DNA, anti-Sm, anti-SS-B, anti-SS-A, and anti-RNP antibodies, were negative. Additionally, anti-neutrophil cytoplasmic antibody was negative. Complement levels were within normal limits, and the C-reactive protein level was elevated at 6.79 mg/dL. Chest radiography showed bilateral patchy infiltrating shadows with consolidation (Fig. 1A). Additionally, chest computed tomography showed multifocal ill-defined nodular opacities in both the lungs. A high number of consolidations and hemoptysis on computed tomography indicated the presence of pulmonary alveolar hemorrhage (Fig. 1B). Echocardiography showed moderate concentric left ventricular hypertrophy and grade 1 left ventricular diastolic dysfunction. Abdominal computed tomography did not reveal adrenal adenoma, hyperplasia, or renal artery stenosis. Additionally, bronchoscopy was not performed. Based on the findings, he was clinically diagnosed with malignant hypertension, and antihypertensive therapy and hemodialysis were started with packed red blood cell transfusion. His blood pressure was reduced with nifedipine, carvedilol, olmesartan, and minoxidil. After blood pressure control, renal biopsy was performed on day 7 of hospitalization to examine the underlying disease. Light microscopy showed up to 15 glomeruli in the renal cortical tissue. The glomeruli showed globally wrinkling, thickening, replication of the basement membrane, and occasional collapsing sclerosis in the glomerular tufts. Additionally, the interlobular and arcuate arteries and arterioles showed intimal fibrosis and multiple layers of periodic acid-Schiff-positive material (‘onion-skin’ changes) (Fig. 2A, B), and two glomeruli showed global glomerulosclerosis. Moreover, the tubules showed atrophy, flattened regenerating epithelium along the basement membrane, and some periodic acid-Schiff-positive proteinaceous casts in the lumen. Electron micrography showed diffuse subendothelial widening and mesangial expansion, and diffuse effacement of the foot processes of podocytes was noted (Fig. 2C). Additionally, red blood cells and fibrin in the arteriolar lumen and platelet aggregation were noted, suggesting fibrin-platelet thrombus consistent with thrombotic microangiopathy (Fig. 2D). Moreover, immunofluorescence analysis showed normal findings. Infiltration shadows in the lungs completely disappeared approximately two weeks after admission. On normalization of blood pressure, his renal function recovered gradually (Fig. 3), and the final hemodialysis was performed on day 26 of hospitalization. His creatinine level reduced to 2.1 mg/dL. He is currently on antihypertensive therapy and has stable renal function.
Discussion
We presented a rare case of malignant hypertension with pulmonary alveolar hemorrhage and rapidly deteriorating renal function that required dialysis. The presentation indicated Goodpasture’s disease, systemic lupus erythematosus, or other systemic vasculitis. The patient did not have fever, arthritis, or peripheral neuropathy; therefore, systemic vasculitis could be excluded. Few cases of malignant hypertension with pulmonary alveolar hemorrhage have been reported previously [2-5]; however, none of the patients needed hemodialysis. Patients who appear to have end-stage renal disease due to malignant nephrosclerosis can recover their renal function [6], as was noted in the present patient. Therefore, early diagnosis and strict blood pressure control are very important to improve renal recovery in patients with malignant hypertension.
Two events may cause malignant hypertension with pulmonary alveolar hemorrhage. First, vascular or endothelial injuries resulting from severe hypertension can cause malignant hypertension. This process may involve not only the mechanical stress of high blood pressure, but also humoral components, such as renin, aldosterone, vasopressin, catecholamines, endothelin [3], and coagulation factors. Second, left ventricular dysfunction resulting from systemic hypertension can cause pulmonary edema, leading to hemorrhage [4].
Any antihypertensive agent can be used to effectively manage malignant hypertension. However, the initial oral therapy should include an arteriolar vasodilator, such as hydralazine, dihydropyridine calcium channel blocker, or minoxidil. Vasodilators may reflexively activate the adrenergic system and cause tachycardia with an increase in cardiac output, which may reduce the hypotensive response. Therefore, treatment with beta-adrenergic blockers is usually required. In the present patient, blood pressure was well controlled with these agents.
We report a rare case of malignant hypertension with pulmonary alveolar hemorrhage and acute kidney injury that needed dialysis. In the present case, it was difficult to determine whether the malignant hypertension was primary or secondary. Renal biopsy was used to exclude the possibility of underlying systemic diseases, such as systemic angiitis syndrome and Goodpasture’s disease [5].